Widespread Doublecortin Expression in the Cerebral Cortex of the Octodon degus
- PMID: 33994960
- PMCID: PMC8116662
- DOI: 10.3389/fnana.2021.656882
Widespread Doublecortin Expression in the Cerebral Cortex of the Octodon degus
Abstract
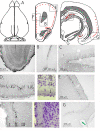
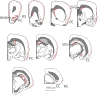
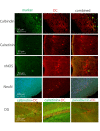
It has been demonstrated that in adulthood rodents show newly born neurons in the subgranular layer (SGL) of the dentate gyrus (DG), and in the subventricular zone (SVZ). The neurons generated in the SVZ migrate through the rostral migratory stream (RMS) to the olfactory bulb. One of the markers of newly generated neurons is doublecortin (DCX). The degu similarly shows significant numbers of DCX-labeled neurons in the SGL, SVZ, and RMS. Further, most of the nuclei of these DCX-expressing neurons are also labeled by proliferating nuclear antigen (PCNA) and Ki67. Finally, whereas in rats and mice DCX-labeled neurons are predominantly present in the SGL and SVZ, with only a few DCX neurons present in piriform cortex, the degu also shows significant numbers of DCX expressing neurons in areas outside of SVZ, DG, and PC. Many areas of neocortex in degu demonstrate DCX-labeled neurons in layer II, and most of these neurons are found in the limbic cortices. The DCX-labeled cells do not stain with NeuN, indicating they are immature neurons.
Keywords: Octodon degus; cerebral cortex; cortical atlas; doublecortin; limbic system.
Copyright © 2021 van Groen, Kadish, Popović, Caballero Bleda, Baño-Otalora, Rol, Madrid and Popović.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Adult neurogenesis in the hedgehog (Erinaceus concolor) and mole (Talpa europaea).Brain Behav Evol. 2010;76(2):128-43. doi: 10.1159/000320944. Epub 2010 Nov 15. Brain Behav Evol. 2010. PMID: 21079393
-
The distribution of doublecortin-immunopositive cells in the brains of four afrotherian mammals: the Hottentot golden mole (Amblysomus hottentotus), the rock hyrax (Procavia capensis), the eastern rock sengi (Elephantulus myurus) and the four-toed sengi (Petrodromus tetradactylus).Brain Behav Evol. 2014;84(3):227-41. doi: 10.1159/000367934. Epub 2014 Nov 5. Brain Behav Evol. 2014. PMID: 25377859
-
Doublecortin-Expressing Neurons in Chinese Tree Shrew Forebrain Exhibit Mixed Rodent and Primate-Like Topographic Characteristics.Front Neuroanat. 2021 Sep 16;15:727883. doi: 10.3389/fnana.2021.727883. eCollection 2021. Front Neuroanat. 2021. PMID: 34602987 Free PMC article.
-
The Distribution of Ki-67 and Doublecortin Immunopositive Cells in the Brains of Three Microchiropteran Species, Hipposideros fuliginosus, Triaenops persicus, and Asellia tridens.Anat Rec (Hoboken). 2016 Nov;299(11):1548-1560. doi: 10.1002/ar.23460. Epub 2016 Aug 26. Anat Rec (Hoboken). 2016. PMID: 27532288
-
Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain.J Neurosci Res. 2004 May 1;76(3):282-95. doi: 10.1002/jnr.20071. J Neurosci Res. 2004. PMID: 15079857
Cited by
-
Generation of a Dcx-CreERT2 knock-in mouse for genetic manipulation of newborn neurons.Genesis. 2024 Feb;62(1):e23584. doi: 10.1002/dvg.23584. Epub 2023 Dec 16. Genesis. 2024. PMID: 38102875
-
Doublecortin-Expressing Neurons in Human Cerebral Cortex Layer II and Amygdala from Infancy to 100 Years Old.Mol Neurobiol. 2023 Jun;60(6):3464-3485. doi: 10.1007/s12035-023-03261-7. Epub 2023 Mar 6. Mol Neurobiol. 2023. PMID: 36879137
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

