Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain
- PMID: 33989741
- PMCID: PMC8319055
- DOI: 10.1016/j.bbi.2021.05.004
Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain
Abstract
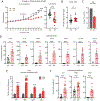
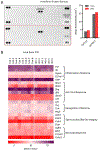
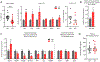
Maternal infection during pregnancy is a known risk factor for offspring mental health disorders. Animal models of maternal immune activation (MIA) have implicated specific cellular and molecular etiologies of psychiatric illness, but most rely on pathogen mimetics. Here, we developed a mouse model of live H3N2 influenza A virus (IAV) infection during pregnancy that induces a robust inflammatory response but is sublethal to both dams and offspring. We observed classic indicators of lung inflammation and severely diminished weight gain in IAV-infected dams. This was accompanied by immune cell infiltration in the placenta and partial breakdown of placental integrity. However, indications of fetal neuroinflammation were absent. Further hallmarks of mimetic-induced MIA, including enhanced circulating maternal IL-17A, were also absent. Respiratory IAV infection did result in an upregulation in intestinal expression of transcription factor RORγt, master regulator of a subset of T lymphocytes, TH17 cells, which are heavily implicated in MIA-induced etiologies. Nonetheless, subsequent augmentation in IL-17A production and concomitant overt intestinal injury was not evident. Our results suggest that mild or moderately pathogenic IAV infection during pregnancy does not inflame the developing fetal brain, and highlight the importance of live pathogen infection models for the study of MIA.
Keywords: Fetal development; Fetal neuroinflammation; Gestational infection; Infection during pregnancy; Influenza A virus; Influenza infection; Maternal immune activation; Maternal inflammation; Maternal viral infection; T helper 17 cells.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Defining the absolute risk of maternal infections on offspring neurodevelopmental outcomes: How to ensure your model is not lost in translation.Brain Behav Immun. 2021 Oct;97:6-7. doi: 10.1016/j.bbi.2021.07.008. Epub 2021 Jul 21. Brain Behav Immun. 2021. PMID: 34298095 Free PMC article. No abstract available.
Similar articles
-
Influenza A virus infection during pregnancy causes immunological changes in gut-associated lymphoid tissues of offspring mice.Am J Physiol Gastrointest Liver Physiol. 2023 Sep 1;325(3):G230-G238. doi: 10.1152/ajpgi.00062.2023. Epub 2023 Jul 11. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 37431584 Free PMC article.
-
At the crux of maternal immune activation: Viruses, microglia, microbes, and IL-17A.Immunol Rev. 2022 Oct;311(1):205-223. doi: 10.1111/imr.13125. Epub 2022 Aug 18. Immunol Rev. 2022. PMID: 35979731 Free PMC article. Review.
-
Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice.Proc Natl Acad Sci U S A. 2020 Oct 6;117(40):24964-24973. doi: 10.1073/pnas.2006905117. Epub 2020 Sep 21. Proc Natl Acad Sci U S A. 2020. PMID: 32958663 Free PMC article.
-
Brain region-specific alterations in gene expression trajectories in the offspring born from influenza A virus infected mice.Brain Behav Immun. 2024 Aug;120:488-498. doi: 10.1016/j.bbi.2024.06.025. Epub 2024 Jun 24. Brain Behav Immun. 2024. PMID: 38925418
-
Influenza Virus Infection during Pregnancy as a Trigger of Acute and Chronic Complications.Viruses. 2022 Dec 7;14(12):2729. doi: 10.3390/v14122729. Viruses. 2022. PMID: 36560733 Free PMC article. Review.
Cited by
-
Role of hormones in the pregnancy and sex-specific outcomes to infections with respiratory viruses.Immunol Rev. 2022 Jul;308(1):123-148. doi: 10.1111/imr.13078. Epub 2022 Apr 4. Immunol Rev. 2022. PMID: 35373371 Free PMC article. Review.
-
Maternal Influenza and Offspring Neurodevelopment.Curr Issues Mol Biol. 2024 Jan 2;46(1):355-366. doi: 10.3390/cimb46010023. Curr Issues Mol Biol. 2024. PMID: 38248325 Free PMC article. Review.
-
Biological Sex and Pregnancy Affect Influenza Pathogenesis and Vaccination.Curr Top Microbiol Immunol. 2023;441:111-137. doi: 10.1007/978-3-031-35139-6_5. Curr Top Microbiol Immunol. 2023. PMID: 37695427
-
Influenza A virus infection during pregnancy causes immunological changes in gut-associated lymphoid tissues of offspring mice.Am J Physiol Gastrointest Liver Physiol. 2023 Sep 1;325(3):G230-G238. doi: 10.1152/ajpgi.00062.2023. Epub 2023 Jul 11. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 37431584 Free PMC article.
-
At the crux of maternal immune activation: Viruses, microglia, microbes, and IL-17A.Immunol Rev. 2022 Oct;311(1):205-223. doi: 10.1111/imr.13125. Epub 2022 Aug 18. Immunol Rev. 2022. PMID: 35979731 Free PMC article. Review.
References
-
- Askovich PS, Sanders CJ, Rosenberger CM, Diercks AH, Dash P, Navarro G, Vogel P, Doherty PC, Thomas PG, Aderem A, 2013. Differential host response, rather than early viral replication efficiency, correlates with pathogenicity caused by influenza viruses. PLoS One 8, e74863. 10.1371/journal.pone.0074863 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

