A Distinct Population of L6 Neurons in Mouse V1 Mediate Cross-Callosal Communication
- PMID: 33987642
- PMCID: PMC8328214
- DOI: 10.1093/cercor/bhab084
A Distinct Population of L6 Neurons in Mouse V1 Mediate Cross-Callosal Communication
Abstract
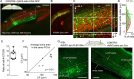
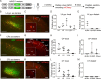
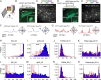
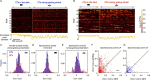
Through the corpus callosum, interhemispheric communication is mediated by callosal projection (CP) neurons. Using retrograde labeling, we identified a population of layer 6 (L6) excitatory neurons as the main conveyer of transcallosal information in the monocular zone of the mouse primary visual cortex (V1). Distinct from L6 corticothalamic (CT) population, V1 L6 CP neurons contribute to an extensive reciprocal network across multiple sensory cortices over two hemispheres. Receiving both local and long-range cortical inputs, they encode orientation, direction, and receptive field information, while are also highly spontaneous active. The spontaneous activity of L6 CP neurons exhibits complex relationships with brain states and stimulus presentation, distinct from the spontaneous activity patterns of the CT population. The anatomical and functional properties of these L6 CP neurons enable them to broadcast visual and nonvisual information across two hemispheres, and thus may play a role in regulating and coordinating brain-wide activity events.
Keywords: callosal projection neurons; corpus callosum; corticothalamic neurons; layer 6; visual cortex.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures

Similar articles
-
Influence of ocular dominance columns and patchy callosal connections on binocularity in lateral striate cortex: Long Evans versus albino rats.J Comp Neurol. 2020 Mar 1;528(4):650-663. doi: 10.1002/cne.24786. Epub 2019 Oct 18. J Comp Neurol. 2020. PMID: 31606892 Free PMC article.
-
Primary visual cortex projections to extrastriate cortices in enucleated and anophthalmic mice.Brain Struct Funct. 2014 Nov;219(6):2051-70. doi: 10.1007/s00429-013-0623-6. Epub 2013 Aug 14. Brain Struct Funct. 2014. PMID: 23942645
-
Laminar distribution and arbor density of two functional classes of thalamic inputs to primary visual cortex.Cell Rep. 2021 Oct 12;37(2):109826. doi: 10.1016/j.celrep.2021.109826. Cell Rep. 2021. PMID: 34644562 Free PMC article.
-
Visual callosal connections: role in visual processing in health and disease.Rev Neurosci. 2014;25(1):113-27. doi: 10.1515/revneuro-2013-0025. Rev Neurosci. 2014. PMID: 24127537 Review.
-
Visual inter-hemispheric processing: constraints and potentialities set by axonal morphology.J Physiol Paris. 1999 Sep-Oct;93(4):271-84. doi: 10.1016/s0928-4257(00)80056-x. J Physiol Paris. 1999. PMID: 10574117 Review.
Cited by
-
Analysis of segmentation ontology reveals the similarities and differences in connectivity onto L2/3 neurons in mouse V1.Sci Rep. 2021 Mar 2;11(1):4983. doi: 10.1038/s41598-021-82353-7. Sci Rep. 2021. PMID: 33654118 Free PMC article.
-
A primary sensory cortical interareal feedforward inhibitory circuit for tacto-visual integration.Nat Commun. 2024 Apr 10;15(1):3081. doi: 10.1038/s41467-024-47459-2. Nat Commun. 2024. PMID: 38594279 Free PMC article.
-
The functional characterization of callosal connections.Prog Neurobiol. 2022 Jan;208:102186. doi: 10.1016/j.pneurobio.2021.102186. Epub 2021 Nov 12. Prog Neurobiol. 2022. PMID: 34780864 Free PMC article. Review.
-
Auditory Corticothalamic Neurons Are Recruited by Motor Preparatory Inputs.Curr Biol. 2021 Jan 25;31(2):310-321.e5. doi: 10.1016/j.cub.2020.10.027. Epub 2020 Nov 5. Curr Biol. 2021. PMID: 33157020 Free PMC article.
-
3D printing-based frugal manufacturing of glass pipettes for minimally invasive delivery of therapeutics to the brain.Neuroprotection. 2023 Sep;1(1):58-65. doi: 10.1002/nep3.20. Epub 2023 Jun 19. Neuroprotection. 2023. PMID: 37771648 Free PMC article.
References
-
- Brown APY, Cossell L, Margrie TW. 2020. Excitatory and inhibitory L2/3 neurons in mouse primary visual cortex are balanced in their input connectivity. bioRxiv 2020.04.21.053504. doi: 10.1101/2020.04.21.053504. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

