Development of an immunochromatographic kit to detect severe acute respiratory syndrome coronavirus 2
- PMID: 33984393
- PMCID: PMC8110331
- DOI: 10.1016/j.jviromet.2021.114183
Development of an immunochromatographic kit to detect severe acute respiratory syndrome coronavirus 2
Abstract
Background: The novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is responsible for the worldwide coronavirus disease-19 (COVID-19) pandemic, starting in late 2019. The standard diagnostic methods to detect SARS-CoV-2 are PCR-based genetic assays. Antigen-antibody-based immunochromatographic assays are alternative methods of detecting this virus. Rapid diagnosis kits to detect SARS-CoV-2 are urgently needed.
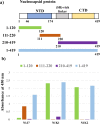
Study design: Three monoclonal antibodies against SARS-CoV-2 nucleocapsid (N) protein were used to develop an antigen-antibody-based immunochromatographic kit to detect SARS-CoV-2. These assays were evaluated using nasopharyngeal swab specimens collected from patients suspected of having COVID-19.
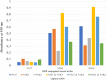
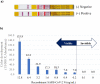
Results: These assays detected recombinant SARS-CoV-2 N protein at concentrations >0.2 ng/mL within 10 min after protein loading, but did not detect the N proteins of Middle East respiratory syndrome coronavirus (MERS-CoV), human coronaviruses OC43 (HCoV-OC43) and 299E (HCoV-229E) and other pathogens causing respiratory infections. Nasopharyngeal swab specimens obtained 1~3, 4~9, and ≥ 10 days after symptom onset from COVID-19 patients diagnosed by RT-PCR showed positivity rates of 100 %, >80 %, and <30 %, respectively.
Conclusions: Kits using this immunochromatographic assay may be a rapid and useful tool for point-of-care diagnosis of COVID-19 when samples are obtained from patients 1~9 days after symptom onset.
Keywords: Coronavirus disease-19 (COVID-19); Immunochromatographic kit; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures
Similar articles
-
Performance and usefulness of a novel automated immunoassay HISCL SARS-CoV-2 Antigen assay kit for the diagnosis of COVID-19.Sci Rep. 2021 Dec 1;11(1):23196. doi: 10.1038/s41598-021-02636-x. Sci Rep. 2021. PMID: 34853366 Free PMC article.
-
Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen.Mikrochim Acta. 2021 May 26;188(6):199. doi: 10.1007/s00604-021-04867-1. Mikrochim Acta. 2021. PMID: 34041585 Free PMC article.
-
Highly specific monoclonal antibodies and epitope identification against SARS-CoV-2 nucleocapsid protein for antigen detection tests.Cell Rep Med. 2021 Jun 15;2(6):100311. doi: 10.1016/j.xcrm.2021.100311. Epub 2021 May 16. Cell Rep Med. 2021. PMID: 34027498 Free PMC article.
-
Human and novel coronavirus infections in children: a review.Paediatr Int Child Health. 2021 Feb;41(1):36-55. doi: 10.1080/20469047.2020.1781356. Epub 2020 Jun 25. Paediatr Int Child Health. 2021. PMID: 32584199 Review.
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
Cited by
-
Development of a colloidal gold immunochromatographic strip for the simultaneous detection of porcine epidemic diarrhea virus and transmissible gastroenteritis virus.Front Microbiol. 2024 Jun 19;15:1418959. doi: 10.3389/fmicb.2024.1418959. eCollection 2024. Front Microbiol. 2024. PMID: 38962124 Free PMC article.
-
Detection of SARS-CoV-2 omicron variants by immunochromatographic kit.Heliyon. 2023 Oct 12;9(10):e20913. doi: 10.1016/j.heliyon.2023.e20913. eCollection 2023 Oct. Heliyon. 2023. PMID: 37876437 Free PMC article.
-
Assessment of an immunochromatographic kit for detection of severe acute respiratory syndrome coronavirus 2 and influenza viruses.J Virol Methods. 2022 Apr;302:114477. doi: 10.1016/j.jviromet.2022.114477. Epub 2022 Jan 22. J Virol Methods. 2022. PMID: 35077720 Free PMC article.
-
Expression of recombinant Omp18 and MOMP of Campylobacter jejuni and the determination of their suitability as antigens for serological diagnosis of campylobacteriosis in animals.Vet World. 2023 Jan;16(1):222-228. doi: 10.14202/vetworld.2023.222-228. Epub 2023 Jan 30. Vet World. 2023. PMID: 36855354 Free PMC article.
References
-
- Kawachi S., Matsushita T., Sato T., et al. Multicenter prospective evaluation of a novel rapid immunochromatographic diagnostic kit specifically detecting influenza A H1N1 2009 virus. J. Clin. Virol. 2011;51(1):68–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

