Context-dependent roles of B cells during intestinal helminth infection
- PMID: 33983946
- PMCID: PMC8118336
- DOI: 10.1371/journal.pntd.0009340
Context-dependent roles of B cells during intestinal helminth infection
Abstract
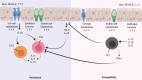
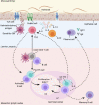
The current approaches to reduce the burden of chronic helminth infections in endemic areas are adequate sanitation and periodic administration of deworming drugs. Yet, resistance against some deworming drugs and reinfection can still rapidly occur even after treatment. A vaccine against helminths would be an effective solution at preventing reinfection. However, vaccines against helminth parasites have yet to be successfully developed. While T helper cells and innate lymphoid cells have been established as important components of the protective type 2 response, the roles of B cells and antibodies remain the most controversial. Here, we review the roles of B cells during intestinal helminth infection. We discuss the potential factors that contribute to the context-specific roles for B cells in protection against diverse intestinal helminth parasite species, using evidence from well-defined murine model systems. Understanding the precise roles of B cells during resistance and susceptibility to helminth infection may offer a new perspective of type 2 protective immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Helminth infection is associated with decreased basophil responsiveness in human beings.J Allergy Clin Immunol. 2012 Jul;130(1):270-2. doi: 10.1016/j.jaci.2012.04.017. Epub 2012 May 16. J Allergy Clin Immunol. 2012. PMID: 22608572 Free PMC article. No abstract available.
-
To B or not to B: B cells and the Th2-type immune response to helminths.Trends Immunol. 2011 Feb;32(2):80-8. doi: 10.1016/j.it.2010.11.005. Epub 2010 Dec 14. Trends Immunol. 2011. PMID: 21159556 Free PMC article. Review.
-
ILC2s-Trailblazers in the Host Response Against Intestinal Helminths.Front Immunol. 2019 Apr 4;10:623. doi: 10.3389/fimmu.2019.00623. eCollection 2019. Front Immunol. 2019. PMID: 31019505 Free PMC article. Review.
-
Current status of medicinal research in helminth diseases.Med Res Rev. 1991 Nov;11(6):581-615. doi: 10.1002/med.2610110603. Med Res Rev. 1991. PMID: 1762474 Review. No abstract available.
-
T Follicular Helper Cell-Derived IL-4 Is Required for IgE Production during Intestinal Helminth Infection.J Immunol. 2017 Jul 1;199(1):244-252. doi: 10.4049/jimmunol.1700141. Epub 2017 May 22. J Immunol. 2017. PMID: 28533444
Cited by
-
In silico design of a polypeptide as a vaccine candidate against ascariasis.Sci Rep. 2023 Mar 2;13(1):3504. doi: 10.1038/s41598-023-30445-x. Sci Rep. 2023. PMID: 36864139 Free PMC article.
-
Targeting helminths: The expanding world of type 2 immune effector mechanisms.J Exp Med. 2023 Oct 2;220(10):e20221381. doi: 10.1084/jem.20221381. Epub 2023 Aug 28. J Exp Med. 2023. PMID: 37638887 Free PMC article. Review.
-
B cell fate mapping reveals their contribution to the memory immune response against helminths.Front Immunol. 2022 Nov 24;13:1016142. doi: 10.3389/fimmu.2022.1016142. eCollection 2022. Front Immunol. 2022. PMID: 36505408 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

