IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer
- PMID: 33981768
- PMCID: PMC8088376
- DOI: 10.1155/2021/5535578
IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer
Abstract
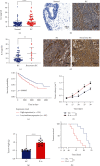
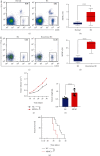
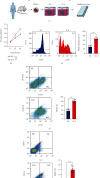
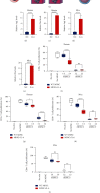
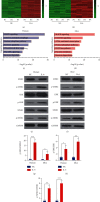
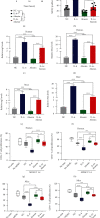
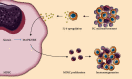
Muscle-invasive bladder cancer (MIBC) is characterized by a highly complex immune environment, which is not well understood. Interleukin-6 (IL-6) is generated and secreted by multifarious types of cells, including tumor cells. This study was aimed at demonstrating that the levels of IL-6 and the number of myeloid-derived suppressor cells (MDSCs), with a positive correlation between them, increased in MIBC tissues, promoting MIBC cell proliferation, especially in patients with recurrence. In coculture analysis, MDSCs, with the stimulation of IL-6, could significantly lower the proliferation ability of CD4+ or CD8+ T lymphocytes. Further, this study demonstrated that IL-6 could upregulate the mitogen-activated protein kinase (MAPK) signaling pathway in MDSCs. The MAPK signaling inhibitor, aloesin, partially reversed the effects of IL-6 on MDSCs. These data suggested that IL-6 promoted MIBC progression by not only accelerating proliferation but also improving the immune suppression ability of MDSCs through activating the MAPK signaling pathway.
Copyright © 2021 Zhong Zheng et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury.Cell Death Dis. 2017 Mar 23;8(3):e2695. doi: 10.1038/cddis.2017.86. Cell Death Dis. 2017. PMID: 28333137 Free PMC article.
-
CD45+CD33lowCD11bdim myeloid-derived suppressor cells suppress CD8+ T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer.Cell Death Dis. 2018 Jul 9;9(7):763. doi: 10.1038/s41419-018-0803-7. Cell Death Dis. 2018. PMID: 29988030 Free PMC article.
-
Stat6 Promotes Intestinal Tumorigenesis in a Mouse Model of Adenomatous Polyposis by Expansion of MDSCs and Inhibition of Cytotoxic CD8 Response.Neoplasia. 2017 Aug;19(8):595-605. doi: 10.1016/j.neo.2017.04.006. Epub 2017 Jun 24. Neoplasia. 2017. PMID: 28654863 Free PMC article.
-
CHBP induces stronger immunosuppressive CD127+ M-MDSC via erythropoietin receptor.Cell Death Dis. 2021 Feb 12;12(2):177. doi: 10.1038/s41419-021-03448-7. Cell Death Dis. 2021. PMID: 33579907 Free PMC article.
-
Cisplatin inhibits the progression of bladder cancer by selectively depleting G-MDSCs: A novel chemoimmunomodulating strategy.Clin Immunol. 2018 Aug;193:60-69. doi: 10.1016/j.clim.2018.01.012. Epub 2018 Feb 2. Clin Immunol. 2018. PMID: 29410331
Cited by
-
Novel therapeutic strategies targeting myeloid-derived suppressor cell immunosuppressive mechanisms for cancer treatment.Explor Target Antitumor Ther. 2024;5(1):187-207. doi: 10.37349/etat.2024.00212. Epub 2024 Feb 28. Explor Target Antitumor Ther. 2024. PMID: 38464388 Free PMC article. Review.
-
Increased CD14+HLA-DR-/low myeloid-derived suppressor cells can be regarded as a biomarker on disease severity and response to therapy in acute coronary syndrome.PeerJ. 2024 Oct 8;12:e18154. doi: 10.7717/peerj.18154. eCollection 2024. PeerJ. 2024. PMID: 39399429 Free PMC article.
-
Myeloid-Derived Suppressor Cells in Bladder Cancer: An Emerging Target.Cells. 2024 Oct 27;13(21):1779. doi: 10.3390/cells13211779. Cells. 2024. PMID: 39513886 Free PMC article. Review.
-
The Urinary Microbiome in Health and Disease: Relevance for Bladder Cancer.Int J Mol Sci. 2024 Jan 31;25(3):1732. doi: 10.3390/ijms25031732. Int J Mol Sci. 2024. PMID: 38339010 Free PMC article. Review.
-
Transcriptome Profiling of Mouse Embryonic Fibroblast Spontaneous Immortalization: A Comparative Analysis.Int J Mol Sci. 2024 Jul 25;25(15):8116. doi: 10.3390/ijms25158116. Int J Mol Sci. 2024. PMID: 39125691 Free PMC article.
References
-
- Mahdavifar N., Ghoncheh M., Pakzad R., Momenimovahed Z., Salehiniya H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pacific Journal of Cancer Prevention. 2016;17(1):381–386. doi: 10.7314/apjcp.2016.17.1.381. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

