Neurotropic influenza A virus infection causes prion protein misfolding into infectious prions in neuroblastoma cells
- PMID: 33980968
- PMCID: PMC8115602
- DOI: 10.1038/s41598-021-89586-6
Neurotropic influenza A virus infection causes prion protein misfolding into infectious prions in neuroblastoma cells
Abstract
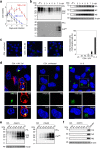
Misfolding of the cellular prion protein, PrPC, into the amyloidogenic isoform, PrPSc, which forms infectious protein aggregates, the so-called prions, is a key pathogenic event in prion diseases. No pathogens other than prions have been identified to induce misfolding of PrPC into PrPSc and propagate infectious prions in infected cells. Here, we found that infection with a neurotropic influenza A virus strain (IAV/WSN) caused misfolding of PrPC into PrPSc and generated infectious prions in mouse neuroblastoma cells through a hit-and-run mechanism. The structural and biochemical characteristics of IAV/WSN-induced PrPSc were different from those of RML and 22L laboratory prions-evoked PrPSc, and the pathogenicity of IAV/WSN-induced prions were also different from that of RML and 22L prions, suggesting IAV/WSN-specific formation of PrPSc and infectious prions. Our current results may open a new avenue for the role of viral infection in misfolding of PrPC into PrPSc and formation of infectious prions.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The first non-prion pathogen identified: neurotropic influenza virus.Prion. 2022 Dec;16(1):1-6. doi: 10.1080/19336896.2021.2015224. Prion. 2022. PMID: 34978525 Free PMC article.
-
Virus Infection, Genetic Mutations, and Prion Infection in Prion Protein Conversion.Int J Mol Sci. 2021 Nov 18;22(22):12439. doi: 10.3390/ijms222212439. Int J Mol Sci. 2021. PMID: 34830321 Free PMC article. Review.
-
Protective role of cytosolic prion protein against virus infection in prion-infected cells.J Virol. 2024 Sep 17;98(9):e0126224. doi: 10.1128/jvi.01262-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194237
-
Central residues in prion protein PrPC are crucial for its conversion into the pathogenic isoform.J Biol Chem. 2022 Sep;298(9):102381. doi: 10.1016/j.jbc.2022.102381. Epub 2022 Aug 13. J Biol Chem. 2022. PMID: 35973512 Free PMC article.
-
Prion Protein is a Novel Modulator of Influenza: Potential Implications for Anti-Influenza Therapeutics.Curr Issues Mol Biol. 2020;37:21-32. doi: 10.21775/cimb.037.021. Epub 2019 Dec 9. Curr Issues Mol Biol. 2020. PMID: 31814573 Review.
Cited by
-
Virus-induced brain pathology and the neuroinflammation-inflammation continuum: the neurochemists view.J Neural Transm (Vienna). 2024 Dec;131(12):1429-1453. doi: 10.1007/s00702-023-02723-5. Epub 2024 Jan 23. J Neural Transm (Vienna). 2024. PMID: 38261034 Free PMC article. Review.
-
Prion Pathogenesis Revealed in a Series of the Special Issues "Prions and Prion Diseases".Int J Mol Sci. 2022 Jun 10;23(12):6490. doi: 10.3390/ijms23126490. Int J Mol Sci. 2022. PMID: 35742934 Free PMC article.
-
Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing.Viruses. 2023 Oct 31;15(11):2198. doi: 10.3390/v15112198. Viruses. 2023. PMID: 38005876 Free PMC article.
-
Enterovirus infection and its relationship with neurodegenerative diseases.Mem Inst Oswaldo Cruz. 2023 Mar 20;118:e220252. doi: 10.1590/0074-02760220252. eCollection 2023. Mem Inst Oswaldo Cruz. 2023. PMID: 36946853 Free PMC article.
-
The first non-prion pathogen identified: neurotropic influenza virus.Prion. 2022 Dec;16(1):1-6. doi: 10.1080/19336896.2021.2015224. Prion. 2022. PMID: 34978525 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

