Mice lacking endogenous TRPV1 express reduced levels of thermogenic proteins and are susceptible to diet-induced obesity and metabolic dysfunction
- PMID: 33977527
- PMCID: PMC8277693
- DOI: 10.1002/1873-3468.14105
Mice lacking endogenous TRPV1 express reduced levels of thermogenic proteins and are susceptible to diet-induced obesity and metabolic dysfunction
Abstract
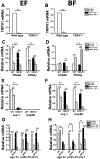
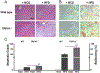
Transient receptor potential vanilloid subfamily 1 (TRPV1) is a non-selective cation channel protein expressed in neuronal and non-neuronal cells. Although TRPV1 is implicated in thermogenesis and diet-induced obesity (DIO), its precise role remains controversial. TRPV1-/- mice are protected from DIO, while TRPV1 activation enhances thermogenesis to prevent obesity. To reconcile this, we fed wild-type and TRPV1-/- mice for 32 weeks with normal chow or a high-fat diet and analyzed the weight gain, metabolic activities, and thermogenic protein expression in white and brown fats. TRPV1-/- mice became obese, exhibited reduced locomotor activity, reduced energy expenditure, enhanced hepatic steatosis, and decreased thermogenic protein expression in adipose tissues. Our data reveal that lack of TRPV1 does not prevent obesity, but rather enhances metabolic dysfunction.
Keywords: TRPV1−/−; adipose tissue; adiposity; fatty liver; metabolism; obesity.
© 2021 Federation of European Biochemical Societies.
Conflict of interest statement
Statement of Competing Interest
The authors have no competing interests.
Figures




Similar articles
-
TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue.Int J Obes (Lond). 2017 May;41(5):739-749. doi: 10.1038/ijo.2017.16. Epub 2017 Jan 20. Int J Obes (Lond). 2017. PMID: 28104916 Free PMC article.
-
Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms.Br J Pharmacol. 2016 Aug;173(15):2369-89. doi: 10.1111/bph.13514. Epub 2016 Jun 21. Br J Pharmacol. 2016. PMID: 27174467 Free PMC article.
-
A role for TRPV1 in influencing the onset of cardiovascular disease in obesity.Hypertension. 2013 Jan;61(1):246-52. doi: 10.1161/HYPERTENSIONAHA.112.201434. Epub 2012 Nov 12. Hypertension. 2013. PMID: 23150506
-
Binding Efficacy and Thermogenic Efficiency of Pungent and Nonpungent Analogs of Capsaicin.Molecules. 2018 Dec 4;23(12):3198. doi: 10.3390/molecules23123198. Molecules. 2018. PMID: 30518154 Free PMC article.
-
Flavonoids, Potential Bioactive Compounds, and Non-Shivering Thermogenesis.Nutrients. 2018 Aug 25;10(9):1168. doi: 10.3390/nu10091168. Nutrients. 2018. PMID: 30149637 Free PMC article. Review.
Cited by
-
Association between Variants of the TRPV1 Gene and Body Composition in Sub-Saharan Africans.Genes (Basel). 2024 Jun 7;15(6):752. doi: 10.3390/genes15060752. Genes (Basel). 2024. PMID: 38927688 Free PMC article.
-
An Overview of the TRP-Oxidative Stress Axis in Metabolic Syndrome: Insights for Novel Therapeutic Approaches.Cells. 2022 Apr 11;11(8):1292. doi: 10.3390/cells11081292. Cells. 2022. PMID: 35455971 Free PMC article. Review.
-
Capsaicin directly promotes adipocyte browning in the chemical compound-induced brown adipocytes converted from human dermal fibroblasts.Sci Rep. 2022 Apr 22;12(1):6612. doi: 10.1038/s41598-022-10644-8. Sci Rep. 2022. PMID: 35459786 Free PMC article.
-
The Role of Cannabidiol in Liver Disease: A Systemic Review.Int J Mol Sci. 2024 Feb 17;25(4):2370. doi: 10.3390/ijms25042370. Int J Mol Sci. 2024. PMID: 38397045 Free PMC article. Review.
-
Targeting the Inflammatory Hallmarks of Obesity-Associated Osteoarthritis: Towards Nutraceutical-Oriented Preventive and Complementary Therapeutic Strategies Based on n-3 Polyunsaturated Fatty Acids.Int J Mol Sci. 2023 May 26;24(11):9340. doi: 10.3390/ijms24119340. Int J Mol Sci. 2023. PMID: 37298291 Free PMC article. Review.
References
-
- Li L et al. (2012). TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice. Pflugers Arch 463, 727–32. - PubMed
-
- Zhang LL et al. (2007). Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res 100, 1063–70. - PubMed
-
- Yoneshiro T, Aita S, Kawai Y, Iwanaga T and Saito M (2012). Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr 95, 845–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

