ARP-T1-associated Bazex-Dupré-Christol syndrome is an inherited basal cell cancer with ciliary defects characteristic of ciliopathies
- PMID: 33972689
- PMCID: PMC8110579
- DOI: 10.1038/s42003-021-02054-9
ARP-T1-associated Bazex-Dupré-Christol syndrome is an inherited basal cell cancer with ciliary defects characteristic of ciliopathies
Abstract
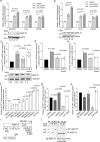
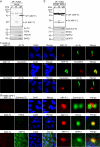
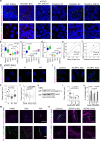
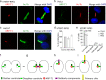
Actin-Related Protein-Testis1 (ARP-T1)/ACTRT1 gene mutations cause the Bazex-Dupré-Christol Syndrome (BDCS) characterized by follicular atrophoderma, hypotrichosis, and basal cell cancer. Here, we report an ARP-T1 interactome (PXD016557) that includes proteins involved in ciliogenesis, endosomal recycling, and septin ring formation. In agreement, ARP-T1 localizes to the midbody during cytokinesis and the basal body of primary cilia in interphase. Tissue samples from ARP-T1-associated BDCS patients have reduced ciliary length. The severity of the shortened cilia significantly correlates with the ARP-T1 levels, which was further validated by ACTRT1 knockdown in culture cells. Thus, we propose that ARP-T1 participates in the regulation of cilia length and that ARP-T1-associated BDCS is a case of skin cancer with ciliopathy characteristics.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Mutations in ACTRT1 and its enhancer RNA elements lead to aberrant activation of Hedgehog signaling in inherited and sporadic basal cell carcinomas.Nat Med. 2017 Oct;23(10):1226-1233. doi: 10.1038/nm.4368. Epub 2017 Sep 4. Nat Med. 2017. PMID: 28869610
-
Germline intergenic duplications at Xq26.1 underlie Bazex-Dupré-Christol basal cell carcinoma susceptibility syndrome.Br J Dermatol. 2022 Dec;187(6):948-961. doi: 10.1111/bjd.21842. Epub 2022 Sep 12. Br J Dermatol. 2022. PMID: 35986704
-
Borderline cognitive level in a family with Bazex-Dupré-Christol syndrome.Am J Med Genet A. 2015 Jul;167(7):1637-43. doi: 10.1002/ajmg.a.37041. Epub 2015 Mar 28. Am J Med Genet A. 2015. PMID: 25820919
-
[Bazex-Dupré-Christol syndrome. Follicular atrophoderma, multiple basal cell carcinomas and hypotrichosis].Hautarzt. 1993 Jun;44(6):385-91. Hautarzt. 1993. PMID: 8335462 Review. German.
-
Sporadic Bazex-Dupré-Christol-like syndrome: early onset basal cell carcinoma, hypohidrosis, hypotrichosis, and prominent milia.Dermatol Surg. 2000 Feb;26(2):152-4. doi: 10.1046/j.1524-4725.2000.99146.x. Dermatol Surg. 2000. PMID: 10691946 Review.
Cited by
-
HaCaT cells as a model system to study primary cilia in keratinocytes.Exp Dermatol. 2022 Aug;31(8):1276-1280. doi: 10.1111/exd.14626. Epub 2022 Jun 23. Exp Dermatol. 2022. PMID: 35708968 Free PMC article.
-
Multidisciplinary approach to Gorlin-Goltz syndrome: from diagnosis to surgical treatment of jawbones.Maxillofac Plast Reconstr Surg. 2022 Jul 18;44(1):25. doi: 10.1186/s40902-022-00355-5. Maxillofac Plast Reconstr Surg. 2022. PMID: 35843976 Free PMC article. Review.
-
Cholesterol in the ciliary membrane as a therapeutic target against cancer.Front Mol Biosci. 2023 Mar 16;10:1160415. doi: 10.3389/fmolb.2023.1160415. eCollection 2023. Front Mol Biosci. 2023. PMID: 37006607 Free PMC article. Review.
-
Multiple Basal Cell Carcinomas in Immunocompetent Patients.Cancers (Basel). 2022 Jun 30;14(13):3211. doi: 10.3390/cancers14133211. Cancers (Basel). 2022. PMID: 35804983 Free PMC article.
References
-
- Bazex, A., Dupre, A. & Christol, B. [Follicular atrophoderma, baso-cellular proliferations and hypotrichosis]. Annal. Dermatologie Syphiligraphie93, 241–254 (1966). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

