Upregulation of miR-20b Protects Against Cerebral Ischemic Stroke by Targeting Thioredoxin Interacting Protein (TXNIP)
- PMID: 33972468
- PMCID: PMC8118756
- DOI: 10.5607/en20046
Upregulation of miR-20b Protects Against Cerebral Ischemic Stroke by Targeting Thioredoxin Interacting Protein (TXNIP)
Abstract
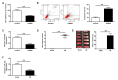
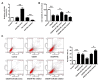
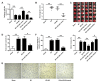
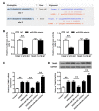
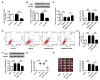
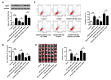
Dysregulation of microRNAs (miRNAs) is involved in abnormal development and pathophysiology in the brain. Although miR-20b plays essential roles in various human diseases, its function in cerebral ischemic stroke remains unclear. A cell model of oxygen glucose deprivation/reoxygenation (OGD/R) and A rat model of middle cerebral artery occlusion/reperfusion (MCAO/R) were constructed. qRT-PCR and western blot were used to evaluate the expression of miR-20b and TXNIP. Cell viability was detected by MTT assay, and cell apoptosis was evaluated by flow cytometry. Targetscan and Starbase were used to predict the potential targets of miR-20b. Luciferase reporter assay was applied to determine the interaction between miR-20b and TXNIP. Rescue experiments were conducted to confirm the functions of miR-20b/TXNIP axis in cerebral ischemic stroke. MiR-20b was significantly downregulated after I/R both in vitro and in vivo. Upregulation of miR-20b inhibited OGD/R-induced neurons apoptosis and attenuated ischemic brain injury in rat model. Bioinformatic prediction suggested that TXNIP might be a target of miR-20b, and luciferase reporter assay revealed that miR-20b negatively regulated TXNIP expression by directly binding to the 3'-UTR of TXNIP. Downregulation of TXNIP inhibited OGD/R-induced neurons apoptosis in vitro and ischemic brain injury in vivo. Rescue experiments indicated that downregulation of TXNIP effectively reversed the effect of miR-20b inhibitor in neurons apoptosis after OGD/R-treatment and ischemic brain injury in a mouse model after MCAO/R-treatment. Our study demonstrated that upregulation of miR-20b protected the brain from ischemic brain injury by targeting TXNIP, extending our understanding of miRNAs in cerebral ischemic stroke.
Keywords: Apoptosis; Cerebral ischemic stroke; Ischemic brain injury; TXNIP; miR-20b.
Figures
Similar articles
-
Inhibition of miR-19a-3p decreases cerebral ischemia/reperfusion injury by targeting IGFBP3 in vivo and in vitro.Biol Res. 2020 Apr 20;53(1):17. doi: 10.1186/s40659-020-00280-9. Biol Res. 2020. PMID: 32312329 Free PMC article.
-
Biliverdin Protects Against Cerebral Ischemia/Reperfusion Injury by Regulating the miR-27a-3p/Rgs1 Axis.Neuropsychiatr Dis Treat. 2021 Apr 22;17:1165-1181. doi: 10.2147/NDT.S300773. eCollection 2021. Neuropsychiatr Dis Treat. 2021. PMID: 33911865 Free PMC article.
-
Upregulation of miR-489-3p Attenuates Cerebral Ischemia/Reperfusion Injury by Targeting Histone Deacetylase 2 (HDAC2).Neuroscience. 2022 Feb 21;484:16-25. doi: 10.1016/j.neuroscience.2021.12.009. Epub 2021 Dec 13. Neuroscience. 2022. PMID: 34914969
-
MiR-139 protects against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced nerve injury through targeting c-Jun to inhibit NLRP3 inflammasome activation.J Stroke Cerebrovasc Dis. 2020 Sep;29(9):105037. doi: 10.1016/j.jstrokecerebrovasdis.2020.105037. Epub 2020 Jun 28. J Stroke Cerebrovasc Dis. 2020. PMID: 32807449
-
MiR-10b-3p Protects Cerebral I/R Injury through Targeting Programmed Cell Death 5 (PDCD5).Crit Rev Eukaryot Gene Expr. 2021;31(6):85-98. doi: 10.1615/CritRevEukaryotGeneExpr.2021039465. Crit Rev Eukaryot Gene Expr. 2021. PMID: 34936294
Cited by
-
Up-regulating microRNA-214-3p relieves hypoxic-ischemic brain damage through inhibiting TXNIP expression.Mol Cell Biochem. 2023 Mar;478(3):597-608. doi: 10.1007/s11010-022-04530-0. Epub 2022 Aug 18. Mol Cell Biochem. 2023. PMID: 35980563
-
Thioredoxin interacting protein, a key molecular switch between oxidative stress and sterile inflammation in cellular response.World J Diabetes. 2021 Dec 15;12(12):1979-1999. doi: 10.4239/wjd.v12.i12.1979. World J Diabetes. 2021. PMID: 35047114 Free PMC article. Review.
-
TXNIP knockdown ameliorates hepatic ischemia/reperfusion injury by inhibiting apoptosis and improving mitochondrial dysfunction via HIF-1α.Mol Cell Biochem. 2024 Jun 13. doi: 10.1007/s11010-024-05037-6. Online ahead of print. Mol Cell Biochem. 2024. PMID: 38872070
-
TXNIP: A Double-Edged Sword in Disease and Therapeutic Outlook.Oxid Med Cell Longev. 2022 Apr 11;2022:7805115. doi: 10.1155/2022/7805115. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35450411 Free PMC article. Review.
References
-
- Xu Q, Deng F, Xing Z, Wu Z, Cen B, Xu S, Zhao Z, Nepomuceno R, Bhuiyan MI, Sun D, Wang QJ, Ji A. Long non-coding RNA C2dat1 regulates CaMKIIδ expression to promote neuronal survival through the NF-κB signaling pathway following cerebral ischemia. Cell Death Dis. 2016;7:e2173. doi: 10.1038/cddis.2016.57. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

