Tumor Spheroids of an Aggressive Form of Central Neurocytoma Have Transit-Amplifying Progenitor Characteristics with Enhanced EGFR and Tumor Stem Cell Signaling
- PMID: 33972466
- PMCID: PMC8118755
- DOI: 10.5607/en21004
Tumor Spheroids of an Aggressive Form of Central Neurocytoma Have Transit-Amplifying Progenitor Characteristics with Enhanced EGFR and Tumor Stem Cell Signaling
Abstract
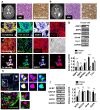
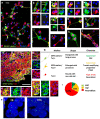
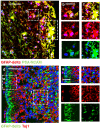
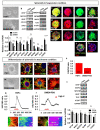
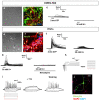
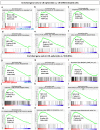
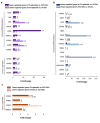
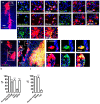
Central neurocytoma (CN) has been known as a benign neuronal tumor. In rare cases, CN undergoes malignant transformation to glioblastomas (GBM). Here we examined its cellular origin by characterizing differentiation potential and gene expression of CN-spheroids. First, we demonstrate that both CN tissue and cultured primary cells recapitulate the hierarchal cellular composition of subventricular zone (SVZ), which is comprised of neural stem cells (NSCs), transit amplifying progenitors (TAPs), and neuroblasts. We then derived spheroids from CN which displayed EGFR+/ MASH+ TAP and BLBP+ radial glial cell (RGC) characteristic, and mitotic neurogenesis and gliogenesis by single spheroids were observed with cycling multipotential cells. CN-spheroids expressed increased levels of pluripotency and tumor stem cell genes such as KLF4 and TPD5L1, when compared to their differentiated cells and human NSCs. Importantly, Gene Set Enrichment Analysis showed that gene sets of GBM-Spheroids, EGFR Signaling, and Packaging of Telomere Ends are enriched in CN-spheroids in comparison with their differentiated cells. We speculate that CN tumor stem cells have TAP and RGC characteristics, and upregulation of EGFR signaling as well as downregulation of eph-ephrin signaling have critical roles in tumorigenesis of CN. And their ephemeral nature of TAPs destined to neuroblasts, might reflect benign nature of CN.
Keywords: Central neurocytoma; Gene Set Enrichment Analysis; Neural stem cell; Radial glia cells; Subventricular zone; Transit-amplifying cells; Tumor spheroids.
Figures


Similar articles
-
The characteristics of neuronal stem cells of central neurocytoma.Neurosurg Clin N Am. 2015 Jan;26(1):31-6. doi: 10.1016/j.nec.2014.09.009. Neurosurg Clin N Am. 2015. PMID: 25432181 Review.
-
Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2- cells as transit-amplifying precursors.Stem Cells. 2009 Jun;27(6):1443-54. doi: 10.1002/stem.74. Stem Cells. 2009. PMID: 19489104
-
Neurocytoma is a tumor of adult neuronal progenitor cells.J Neurosci. 2006 Nov 29;26(48):12544-55. doi: 10.1523/JNEUROSCI.0829-06.2006. J Neurosci. 2006. PMID: 17135416 Free PMC article.
-
Interaction between DLX2 and EGFR regulates proliferation and neurogenesis of SVZ precursors.Mol Cell Neurosci. 2009 Dec;42(4):308-14. doi: 10.1016/j.mcn.2009.08.003. Epub 2009 Aug 14. Mol Cell Neurosci. 2009. PMID: 19683576
-
Shades of gray: The delineation of marker expression within the adult rodent subventricular zone.Prog Neurobiol. 2013 Dec;111:1-16. doi: 10.1016/j.pneurobio.2013.07.003. Epub 2013 Aug 28. Prog Neurobiol. 2013. PMID: 23994259 Review.
Cited by
-
Cancer Stem Cells in Tumours of the Central Nervous System in Children: A Comprehensive Review.Cancers (Basel). 2023 Jun 11;15(12):3154. doi: 10.3390/cancers15123154. Cancers (Basel). 2023. PMID: 37370764 Free PMC article. Review.
-
Central neurocytoma exhibits radial glial cell signatures with FGFR3 hypomethylation and overexpression.Exp Mol Med. 2024 Apr;56(4):975-986. doi: 10.1038/s12276-024-01204-3. Epub 2024 Apr 12. Exp Mol Med. 2024. PMID: 38609519 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

