Influence of fine particulate matter and its pure particulate fractions on pulmonary immune cells and cytokines in mice
- PMID: 33968192
- PMCID: PMC8097186
- DOI: 10.3892/etm.2021.10094
Influence of fine particulate matter and its pure particulate fractions on pulmonary immune cells and cytokines in mice
Abstract
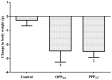
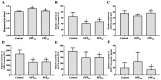
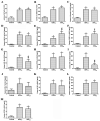
Particulate matter with a diameter ≤2.5 µm (PM2.5) has a complex composition and has been associated with the incidence of cardiopulmonary disease and premature death in humans. However, whether pure particulate fractions of PM2.5 (PPP2.5), which are composed primarily of carbon, are responsible for the toxicity caused by ambient particulate matter (original PM2.5 particles, OPP2.5) is currently unclear. The present study assessed the acute toxic effects of OPP2.5 sampled in Beijing, China and of its PPP2.5 fraction in male BALB/c mice. The mice were intratracheally instilled with a single dose of aerosolized OPP2.5 or PPP2.5. Blood, lungs and bronchoalveolar lavage fluid were collected after 24 h for histopathology, flow cytometry and the measurement of pro-inflammatory cytokines/chemokines and other biochemical factors. Both OPP2.5 and PPP2.5 caused acute toxicity, particularly inflammatory responses, including an increase in the levels of pro-inflammatory cytokines and an accumulation of numerous immune cells in the lungs. OPP2.5 induced a stronger inflammatory response than PPP2.5. The complex components adsorbed into the solid core granules of OPP2.5 and the granules themselves contributed to the toxic effects.
Keywords: acute toxicity; histopathology; immune system; inflammation; particulate matter.
Copyright: © Jiao et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Effects of concentrated ambient particles on normal and hypersecretory airways in rats.Res Rep Health Eff Inst. 2004 Aug;(120):1-68; discussion 69-79. Res Rep Health Eff Inst. 2004. PMID: 15543855
-
Differential pulmonary effects of wintertime California and China particulate matter in healthy young mice.Toxicol Lett. 2017 Aug 15;278:1-8. doi: 10.1016/j.toxlet.2017.07.853. Epub 2017 Jul 8. Toxicol Lett. 2017. PMID: 28698096 Free PMC article.
-
Urban particulate matter in Beijing, China, enhances allergen-induced murine lung eosinophilia.Inhal Toxicol. 2010 Aug;22(9):709-18. doi: 10.3109/08958371003631608. Inhal Toxicol. 2010. PMID: 20560731
-
Early-life exposure to three size-fractionated ultrafine and fine atmospheric particulates in Beijing exacerbates asthma development in mature mice.Part Fibre Toxicol. 2018 Mar 14;15(1):13. doi: 10.1186/s12989-018-0249-1. Part Fibre Toxicol. 2018. PMID: 29540228 Free PMC article.
-
Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms.J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008 Oct-Dec;26(4):339-62. doi: 10.1080/10590500802494538. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008. PMID: 19034792 Review.
Cited by
-
A comprehensive understanding of ambient particulate matter and its components on the adverse health effects based from epidemiological and laboratory evidence.Part Fibre Toxicol. 2022 Nov 29;19(1):67. doi: 10.1186/s12989-022-00507-5. Part Fibre Toxicol. 2022. PMID: 36447278 Free PMC article. Review.
-
Particulate matters (PM2.5, PM10) and the risk of depression among middle-aged and older population: analysis of the Korean Longitudinal Study of Aging (KLoSA), 2016-2020 in South Korea.Environ Health. 2024 Jan 3;23(1):4. doi: 10.1186/s12940-023-01043-1. Environ Health. 2024. PMID: 38172858 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
