Immune Response of Indian Preterm Infants to Pentavalent Vaccine Varies With Component Antigens and Gestational Age
- PMID: 33968011
- PMCID: PMC8102823
- DOI: 10.3389/fimmu.2021.592731
Immune Response of Indian Preterm Infants to Pentavalent Vaccine Varies With Component Antigens and Gestational Age
Abstract
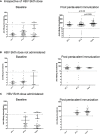
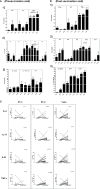
Childhood vaccination plays critical role in protecting infants from several dreaded diseases. Of the global 15 million preterm (PT) infants with compromised immune system born annually, India contributes to >3.5 million. Generation of adequate vaccine-induced immune response needs to be ensured of their protection. Immune response of Indian PT (n = 113) and full-term (FT, n = 80) infants to pentavalent vaccine administered as per the national recommendation was studied. Antibody titers against component antigens of pentavalent vaccine, immune cells profiling (T and B cells, monocytes and dendritic cells) and plasma cytokines were determined pre- and post-vaccination. Additionally, cell-mediated recall immune responses to pentavalent antigens were evaluated after short time antigenic exposure to infant PBMCs. Irrespective of gestational age (GA), all the infants developed adequate antibody response against tetanus, diphtheria, and protective but lower antibody levels for Haemophilus influenzae type-b and hepatitis B in preterm infants. Lower (~74%) protective antibody response to pertussis was independent of gestational age. PT-infants exhibited lower frequencies of CD4 T cells/dendritic cells/monocytes, increased plasma IL-10 levels and lower proliferation of central and effector memory T cells than in term-infants. Proliferative central memory response of FT-infants without anti-pertussis antibodies suggests protection from subsequent infection. Responder/non-responder PT-infants lacked immunological memory and could be infected with Bordetella. For hepatitis B, the recall response was gestational age-dependent and antibody status-independent. Humoral/cellular immune responses of PT-infants were dependent on the type of the immunogen. Preterm infants born before 32 weeks of gestation may need an extra dose of pentavalent vaccine for long lived robust immune response.
Keywords: immune response; immunological memory; pentavalent vaccine; preterm birth; recall immune responses.
Copyright © 2021 Kulkarni-Munje, Malshe, Palkar, Amlekar, Lalwani, Mishra and Arankalle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Antipolyribosyl ribitol phosphate response of premature infants to primary and booster vaccination with a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio virus/Haemophilus influenzae type b vaccine.Pediatrics. 2007 Jan;119(1):e179-85. doi: 10.1542/peds.2005-2907. Epub 2006 Dec 4. Pediatrics. 2007. PMID: 17145903
-
Response of preterm newborns to immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B virus-inactivated polio and Haemophilus influenzae type b vaccine: first experiences and solutions to a serious and sensitive issue.Pediatrics. 2005 Dec;116(6):1292-8. doi: 10.1542/peds.2004-2336. Pediatrics. 2005. PMID: 16322149
-
Association of Routine Infant Vaccinations With Antibody Levels Among Preterm Infants.JAMA. 2020 Sep 15;324(11):1068-1077. doi: 10.1001/jama.2020.12316. JAMA. 2020. PMID: 32930758 Free PMC article.
-
Immunization of preterm infants with GSK's hexavalent combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine: A review of safety and immunogenicity.Vaccine. 2018 Feb 8;36(7):986-996. doi: 10.1016/j.vaccine.2018.01.005. Epub 2018 Jan 12. Vaccine. 2018. PMID: 29336924 Review.
-
Spotlight on DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa).BioDrugs. 2010 Oct 1;24(5):299-302. doi: 10.2165/11206690-000000000-00000. BioDrugs. 2010. PMID: 20795752 Review.
Cited by
-
Penta- and hexavalent vaccination of extremely and very-to-moderate preterm infants born at less than 34 weeks and/or under 1500 g: A systematic literature review.Hum Vaccin Immunother. 2023 Dec 31;19(1):2191575. doi: 10.1080/21645515.2023.2191575. Hum Vaccin Immunother. 2023. PMID: 37076111 Free PMC article.
-
A retrospective study of hepatitis B vaccination in preterm birth and low birth weight infants born to hepatitis B surface antigen-positive mothers: Time to close the policy-practice gap.Hum Vaccin Immunother. 2022 Dec 30;18(7):2155390. doi: 10.1080/21645515.2022.2155390. Epub 2022 Dec 14. Hum Vaccin Immunother. 2022. PMID: 36514905 Free PMC article. Review.
-
Interleukin-2 gene methylation levels and interleukin-2 levels associated with environmental exposure as risk biomarkers for preterm birth.Croat Med J. 2023 Oct 31;64(5):320-328. doi: 10.3325/cmj.2023.64.320. Croat Med J. 2023. PMID: 37927185 Free PMC article.
-
Immunization of preterm infants: current evidence and future strategies to individualized approaches.Semin Immunopathol. 2022 Nov;44(6):767-784. doi: 10.1007/s00281-022-00957-1. Epub 2022 Aug 3. Semin Immunopathol. 2022. PMID: 35922638 Free PMC article. Review.
References
-
- UNICEF . Levels & Trends in Child Mortality, Report 2014 (2014). Available at: http://www.who.int/maternal_child_adolescent/documents/levels_trends_chi....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

