NEDD4 regulates ubiquitination and stability of the cell adhesion molecule IGPR-1 via lysosomal pathway
- PMID: 33962630
- PMCID: PMC8103646
- DOI: 10.1186/s12929-021-00731-9
NEDD4 regulates ubiquitination and stability of the cell adhesion molecule IGPR-1 via lysosomal pathway
Abstract
Background: The cell adhesion molecule IGPR-1 regulates various critical cellular processes including, cell-cell adhesion, mechanosensing and autophagy and plays important roles in angiogenesis and tumor growth; however, the molecular mechanism governing the cell surface levels of IGPR-1 remains unknown.
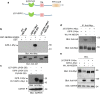
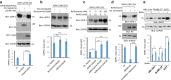
Results: In the present study, we used an in vitro ubiquitination assay and identified ubiquitin E3 ligase NEDD4 and the ubiquitin conjugating enzyme UbcH6 involved in the ubiquitination of IGPR-1. In vitro GST-pulldown and in vivo co-immunoprecipitation assays demonstrated that NEDD4 binds to IGPR-1. Over-expression of wild-type NEDD4 downregulated IGPR-1 and deletion of WW domains (1-4) of NEDD4 revoked its effects on IGPR-1. Knockdown of NEDD4 increased IGPR-1 levels in A375 melanoma cells. Deletion of 57 amino acids encompassing the polyproline rich (PPR) motifs on the C-terminus of IGPR-1 nullified its binding with NEDD4. Furthermore, we demonstrate that NEDD4 promotes K48- and K63-dependent polyubiquitination of IGPR-1. The NEDD4-mediated polyubiquitination of IGPR-1 stimulates lysosomal-dependent degradation of IGPR-1 as the treatment of cells with the lysosomal inhibitors, bafilomycine or ammonium chloride increased IGPR-1 levels ectopically expressed in HEK-293 cells and in multiple endogenously IGPR-1 expressing human skin melanoma cell lines.
Conclusions: NEDD4 ubiquitin E3 ligase binds to and mediates polyubiquitination of IGPR-1 leading to its lysosomal-dependent degradation. NEDD4 is a key regulator of IGPR-1 expression with implication in the therapeutic targeting of IGPR-1 in human cancers.
Keywords: CD28H; IGPR-1; K48-linked ubiquitination; K63-linked ubiquitination; Lysosomal degradation; Melanoma; NEDD4; TMIGD2; UbcH6; Ubiquitination.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
E3 ubiquitin ligase Nedd4 inhibits AP-1 activity and TNF-α production through targeting p38α for polyubiquitination and subsequent degradation.Sci Rep. 2017 Jul 3;7(1):4521. doi: 10.1038/s41598-017-04072-2. Sci Rep. 2017. PMID: 28674435 Free PMC article.
-
The E3 ubiquitin ligase NEDD4 mediates cell migration signaling of EGFR in lung cancer cells.Mol Cancer. 2018 Feb 19;17(1):24. doi: 10.1186/s12943-018-0784-2. Mol Cancer. 2018. PMID: 29455656 Free PMC article.
-
HECT E3 ubiquitin ligase Nedd4-1 ubiquitinates ACK and regulates epidermal growth factor (EGF)-induced degradation of EGF receptor and ACK.Mol Cell Biol. 2010 Mar;30(6):1541-54. doi: 10.1128/MCB.00013-10. Epub 2010 Jan 19. Mol Cell Biol. 2010. PMID: 20086093 Free PMC article.
-
The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives.Mol Med. 2023 Mar 14;29(1):34. doi: 10.1186/s10020-023-00628-3. Mol Med. 2023. PMID: 36918822 Free PMC article. Review.
-
NEDD4 E3 ligase: Functions and mechanism in human cancer.Semin Cancer Biol. 2020 Dec;67(Pt 2):92-101. doi: 10.1016/j.semcancer.2020.03.006. Epub 2020 Apr 11. Semin Cancer Biol. 2020. PMID: 32171886 Review.
Cited by
-
Role of K63-linked ubiquitination in cancer.Cell Death Discov. 2022 Oct 6;8(1):410. doi: 10.1038/s41420-022-01204-0. Cell Death Discov. 2022. PMID: 36202787 Free PMC article. Review.
-
PRMT4-mediated arginine methylation promotes tyrosine phosphorylation of VEGFR-2 and regulates filopodia protrusions.iScience. 2022 Jul 11;25(8):104736. doi: 10.1016/j.isci.2022.104736. eCollection 2022 Aug 19. iScience. 2022. PMID: 35942094 Free PMC article.
-
The Role of NEDD4 E3 Ubiquitin-Protein Ligases in Parkinson's Disease.Genes (Basel). 2022 Mar 14;13(3):513. doi: 10.3390/genes13030513. Genes (Basel). 2022. PMID: 35328067 Free PMC article. Review.
-
NEDD4 and NEDD4L: Ubiquitin Ligases Closely Related to Digestive Diseases.Biomolecules. 2024 May 13;14(5):577. doi: 10.3390/biom14050577. Biomolecules. 2024. PMID: 38785984 Free PMC article. Review.
-
Shikonin blocks CAF-induced TNBC metastasis by suppressing mitochondrial biogenesis through GSK-3β/NEDD4-1 mediated phosphorylation-dependent degradation of PGC-1α.J Exp Clin Cancer Res. 2024 Jun 27;43(1):180. doi: 10.1186/s13046-024-03101-z. J Exp Clin Cancer Res. 2024. PMID: 38937832 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

