Potential mechanism of Achyranthis bidentatae radix plus semen vaccariae granules in the treatment of diabetes mellitus-induced erectile dysfunction in rats utilizing combined experimental model and network pharmacology
- PMID: 33962551
- PMCID: PMC8118505
- DOI: 10.1080/13880209.2021.1920621
Potential mechanism of Achyranthis bidentatae radix plus semen vaccariae granules in the treatment of diabetes mellitus-induced erectile dysfunction in rats utilizing combined experimental model and network pharmacology
Abstract
Context: Achyranthes bidentata Blume (Amaranthaceae) (ABR) and semen vaccariae (SV) are used commonly in the clinical treatment of erectile dysfunction in males with diabetes mellitus (DMED) to strengthen the kidney and promote blood circulation, and often achieve good curative effects.
Objective: Explore mechanistic details of ABR + SV treatment against DMED.
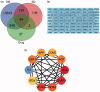
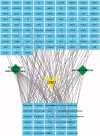
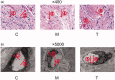
Materials and methods: Prediction of key targets by network pharmacology. A rat model of DM was established by streptozotocin injection (55 mg/kg). Apomorphine (100 μg/kg) was injected into rats to screen the DMED model. Group C (n = 6) and group M (n = 6) were gavaged with deionized water; group T (n = 6) was given Achyranthis bidentatae radix-semen vaccariae granule suspension (2.5 g/kg). It lasted 8 weeks. Real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting (WB) were used to measure the expression of tissue-related proteins and mRNA.
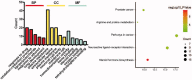
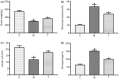
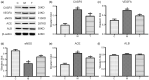
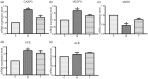
Results: The predicted key targets are albumin (ALB), caspase-3 (CASP3), vascular endothelial growth factor A (VEGFA), angiotensin-converting enzyme (ACE), and endothelial nitric oxide synthase (eNOS). Compared with the M group (0.52 ± 0.04; 0.50 ± 0.03; 0.49 ± 0.02; 0.23 ± 0.03), CASP3, VEGFA, and ACE protein expression reduced in the T group (0.39 ± 0.06; 0.34 ± 0.03; 0.39 ± 0.03), and eNOS protein expression increased (0.34 ± 0.03).
Conclusion: ABR + SV can improve erectile function in DMED rats. This study provides a potential mechanism for the treatment of DMED with ABR + SV and can benefit from more patients.
Keywords: Endocrine diseases; biological network; penis; traditional Chinese medicine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Network Pharmacology Analysis of the Effects of Achyranthis Bidentatae Radix Plus Semen Vaccariae on Migraine-induced Erectile Dysfunction.Comb Chem High Throughput Screen. 2022;25(9):1474-1487. doi: 10.2174/1386207324666210628105233. Comb Chem High Throughput Screen. 2022. PMID: 34182905
-
Leech-Centipede Granules Suppress EndMT to Improve Erectile Dysfunction in Rats with Diabetes Mellitus via TGF-β/Smad Pathway.Chin J Integr Med. 2023 Jan;29(1):28-36. doi: 10.1007/s11655-022-3728-z. Epub 2022 Dec 21. Chin J Integr Med. 2023. PMID: 36542225
-
Rapamycin Supplementation May Ameliorate Erectile Function in Rats With Streptozotocin-Induced Type 1 Diabetes by Inducing Autophagy and Inhibiting Apoptosis, Endothelial Dysfunction, and Corporal Fibrosis.J Sex Med. 2018 Sep;15(9):1246-1259. doi: 10.1016/j.jsxm.2018.07.013. J Sex Med. 2018. PMID: 30224017
-
Melatonin prevents deterioration of erectile function in streptozotocin-induced diabetic rats via sirtuin-1 expression.Andrologia. 2020 Oct;52(9):e13639. doi: 10.1111/and.13639. Epub 2020 Jun 1. Andrologia. 2020. PMID: 32478903 Review.
-
Mesenchymal Stem Cells Treatment for Erectile Dysfunction in Diabetic Rats.Sex Med Rev. 2020 Jan;8(1):114-121. doi: 10.1016/j.sxmr.2019.09.003. Epub 2019 Oct 22. Sex Med Rev. 2020. PMID: 31653438 Review.
Cited by
-
Traditional chinese medicine to prevent and treat diabetic erectile dysfunction.Front Pharmacol. 2022 Sep 21;13:956173. doi: 10.3389/fphar.2022.956173. eCollection 2022. Front Pharmacol. 2022. PMID: 36210810 Free PMC article. Review.
-
Baicalin Rescues Cognitive Dysfunction, Mitigates Neurodegeneration, and Exerts Anti-Epileptic Effects Through Activating TLR4/MYD88/Caspase-3 Pathway in Rats.Drug Des Devel Ther. 2021 Jul 20;15:3163-3180. doi: 10.2147/DDDT.S314076. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34321866 Free PMC article.
References
-
- Chen GH, Sun DL, Jin BF, Zhang XD, Chen B.. 2016. Analysis of Jin Baofang's experience in treating erectile dysfunction. Liaoning J Tradit Chin Med. 43(01):141–143.
-
- Chen W, Gong L, Guo Z, Wang W, Zhang H, Liu X, Yu S, Xiong L, Luo J.. 2013. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Mole Plant. 6:1769–1780. - PubMed
-
- De Young L, Yu D, Bateman RM, Brock GB.. 2004. Oxidative stress and antioxidant therapy: their bimpact in diabetes-associated erectile dysfunction. J Androl. 25(5):830–836. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
