From Conception to Development: Investigating PROTACs Features for Improved Cell Permeability and Successful Protein Degradation
- PMID: 33959589
- PMCID: PMC8093871
- DOI: 10.3389/fchem.2021.672267
From Conception to Development: Investigating PROTACs Features for Improved Cell Permeability and Successful Protein Degradation
Abstract
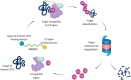
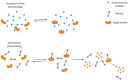
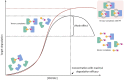
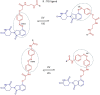
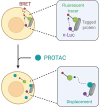
Proteolysis Targeting Chimeras (PROTACs) are heterobifunctional degraders that specifically eliminate targeted proteins by hijacking the ubiquitin-proteasome system (UPS). This modality has emerged as an orthogonal approach to the use of small-molecule inhibitors for knocking down classic targets and disease-related proteins classified, until now, as "undruggable." In early 2019, the first targeted protein degraders reached the clinic, drawing attention to PROTACs as one of the most appealing technology in the drug discovery landscape. Despite these promising results, PROTACs are often affected by poor cellular permeability due to their high molecular weight (MW) and large exposed polar surface area (PSA). Herein, we report a comprehensive record of PROTAC design, pharmacology and thermodynamic challenges and solutions, as well as some of the available strategies to enhance cellular uptake, including suggestions of promising biological tools for the in vitro evaluation of PROTACs permeability toward successful protein degradation.
Keywords: PROTAC technology; cell permeability; drug discovery; protein degradation; proteolysis targeting chimeras; ubiquitin-proteasome system.
Copyright © 2021 Cecchini, Pannilunghi, Tardy and Scapozza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
PROTACs: Current Trends in Protein Degradation by Proteolysis-Targeting Chimeras.BioDrugs. 2022 Sep;36(5):609-623. doi: 10.1007/s40259-022-00551-9. Epub 2022 Sep 13. BioDrugs. 2022. PMID: 36098871 Review.
-
PROTACs: An Emerging Targeting Technique for Protein Degradation in Drug Discovery.Bioessays. 2018 Apr;40(4):e1700247. doi: 10.1002/bies.201700247. Epub 2018 Feb 23. Bioessays. 2018. PMID: 29473971 Review.
-
Linkers as Game-changers in PROTAC Technology: Emphasizing General Trends in PROTAC Pharmacokinetics for their Rational Design.Chimia (Aarau). 2022 Apr 27;76(4):341-345. doi: 10.2533/chimia.2022.341. Chimia (Aarau). 2022. PMID: 38069776
-
Degraders upgraded: the rise of PROTACs in hematological malignancies.Blood. 2024 Mar 28;143(13):1218-1230. doi: 10.1182/blood.2023022993. Blood. 2024. PMID: 38170175 Review.
-
Proteolysis-targeting chimera (PROTAC) delivery system: advancing protein degraders towards clinical translation.Chem Soc Rev. 2022 Jul 4;51(13):5330-5350. doi: 10.1039/d1cs00762a. Chem Soc Rev. 2022. PMID: 35713468 Free PMC article. Review.
Cited by
-
Revolutionizing Drug Targeting Strategies: Integrating Artificial Intelligence and Structure-Based Methods in PROTAC Development.Pharmaceuticals (Basel). 2023 Nov 24;16(12):1649. doi: 10.3390/ph16121649. Pharmaceuticals (Basel). 2023. PMID: 38139776 Free PMC article. Review.
-
Targeted Degradation of Protein Kinase A via a Stapled Peptide PROTAC.ACS Chem Biol. 2024 Sep 20;19(9):1888-1895. doi: 10.1021/acschembio.4c00237. Epub 2024 Aug 13. ACS Chem Biol. 2024. PMID: 39137166 Free PMC article.
-
Aberrant SKP1 Expression: Diverse Mechanisms Impacting Genome and Chromosome Stability.Front Cell Dev Biol. 2022 Mar 8;10:859582. doi: 10.3389/fcell.2022.859582. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35345853 Free PMC article. Review.
-
Targeted Protein Degradation: Principles and Applications of the Proteasome.Cells. 2023 Jul 13;12(14):1846. doi: 10.3390/cells12141846. Cells. 2023. PMID: 37508510 Free PMC article. Review.
-
Targeting Protein Degradation Pathways in Tumors: Focusing on their Role in Hematological Malignancies.Cancers (Basel). 2022 Aug 3;14(15):3778. doi: 10.3390/cancers14153778. Cancers (Basel). 2022. PMID: 35954440 Free PMC article. Review.
References
-
- Alex A., Millan D. S., Perez M., Wakenhut F., Whitlock G. A. (2011). Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space. MedChemComm 2, 669–674. 10.1039/c1md00093d - DOI
-
- Artursson P., Magnusson C. (1990). Epithelial transport of drugs in cell culture. II: effect of extracellular calcium concentration on the paracellular transport of drugs of different lipophilicities across monolayers of intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 79, 595–600. 10.1002/jps.2600790710 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

