Unraveling the Complex Interplay Between Transcription Factors and Signaling Molecules in Thyroid Differentiation and Function, From Embryos to Adults
- PMID: 33959098
- PMCID: PMC8095082
- DOI: 10.3389/fendo.2021.654569
Unraveling the Complex Interplay Between Transcription Factors and Signaling Molecules in Thyroid Differentiation and Function, From Embryos to Adults
Abstract
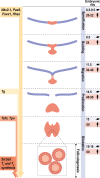
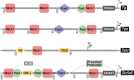
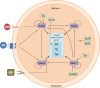
Thyroid differentiation of progenitor cells occurs during embryonic development and in the adult thyroid gland, and the molecular bases of these complex and finely regulated processes are becoming ever more clear. In this Review, we describe the most recent advances in the study of transcription factors, signaling molecules and regulatory pathways controlling thyroid differentiation and development in the mammalian embryo. We also discuss the maintenance of the adult differentiated phenotype to ensure the biosynthesis of thyroid hormones. We will focus on endoderm-derived thyroid epithelial cells, which are responsible for the formation of the thyroid follicle, the functional unit of the thyroid gland. The use of animal models and pluripotent stem cells has greatly aided in providing clues to the complicated puzzle of thyroid development and function in adults. The so-called thyroid transcription factors - Nkx2-1, Foxe1, Pax8 and Hhex - were the first pieces of the puzzle identified in mice. Other transcription factors, either acting upstream of or directly with the thyroid transcription factors, were subsequently identified to, almost, complete the puzzle. Among them, the transcription factors Glis3, Sox9 and the cofactor of the Hippo pathway Taz, have emerged as important players in thyroid differentiation and development. The involvement of signaling molecules increases the complexity of the puzzle. In this context, the importance of Bmps, Fgfs and Shh signaling at the onset of development, and of TSH, IGF1 and TGFβ both at the end of terminal differentiation in embryos and in the adult thyroid, are well recognized. All of these aspects are covered herein. Thus, readers will be able to visualize the puzzle of thyroid differentiation with most - if not all - of the pieces in place.
Keywords: development; differentiation; signaling pathways; thyroid; transcription factors.
Copyright © 2021 López-Márquez, Carrasco-López, Fernández-Méndez and Santisteban.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures

Similar articles
-
Sox9 is involved in the thyroid differentiation program and is regulated by crosstalk between TSH, TGFβ and thyroid transcription factors.Sci Rep. 2022 Feb 9;12(1):2144. doi: 10.1038/s41598-022-06004-1. Sci Rep. 2022. PMID: 35140269 Free PMC article.
-
An integrated regulatory network controlling survival and migration in thyroid organogenesis.Dev Biol. 2004 Dec 15;276(2):464-75. doi: 10.1016/j.ydbio.2004.08.048. Dev Biol. 2004. PMID: 15581879
-
Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells.Thyroid. 2013 Apr;23(4):385-91. doi: 10.1089/thy.2012.0644. Epub 2013 Mar 18. Thyroid. 2013. PMID: 23360087 Free PMC article.
-
Thyroid transcription factors in development, differentiation and disease.Nat Rev Endocrinol. 2015 Jan;11(1):29-42. doi: 10.1038/nrendo.2014.186. Epub 2014 Oct 28. Nat Rev Endocrinol. 2015. PMID: 25350068 Review.
-
From Endoderm to Progenitors: An Update on the Early Steps of Thyroid Morphogenesis in the Zebrafish.Front Endocrinol (Lausanne). 2021 Jun 4;12:664557. doi: 10.3389/fendo.2021.664557. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34149617 Free PMC article. Review.
Cited by
-
Autoimmune hypothyroidism GWAS reveals independent autoimmune and thyroid-specific contributions and an inverse relation with cancer risk.Res Sq [Preprint]. 2024 Jul 8:rs.3.rs-4626646. doi: 10.21203/rs.3.rs-4626646/v1. Res Sq. 2024. PMID: 39041034 Free PMC article. Preprint.
-
A review of complex hormone regulation in thyroid cancer: novel insights beyond the hypothalamus-pituitary-thyroid axis.Front Endocrinol (Lausanne). 2024 Jul 22;15:1419913. doi: 10.3389/fendo.2024.1419913. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39104813 Free PMC article. Review.
-
Immunohistochemical Evaluation of BARX1, DLX4, FOXE1, HOXB3, and MSX2 in Nonsyndromic Cleft Affected Tissue.Acta Med Litu. 2022;29(2):271-294. doi: 10.15388/Amed.2022.29.2.13. Epub 2022 Jun 29. Acta Med Litu. 2022. PMID: 37733420 Free PMC article.
-
GLIS3 expression in the thyroid gland in relation to TSH signaling and regulation of gene expression.Cell Mol Life Sci. 2024 Jan 28;81(1):65. doi: 10.1007/s00018-024-05113-6. Cell Mol Life Sci. 2024. PMID: 38281222 Free PMC article.
-
GWAS of thyroid dysgenesis identifies a risk locus at 2q33.3 linked to regulation of Wnt signaling.Hum Mol Genet. 2022 Nov 28;31(23):3967-3974. doi: 10.1093/hmg/ddac093. Hum Mol Genet. 2022. PMID: 35535691 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

