Mitochondrial calcium uniporter is essential for hearing and hair cell preservation in congenic FVB/NJ mice
- PMID: 33958614
- PMCID: PMC8102556
- DOI: 10.1038/s41598-021-88841-0
Mitochondrial calcium uniporter is essential for hearing and hair cell preservation in congenic FVB/NJ mice
Abstract
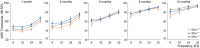
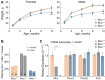
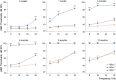
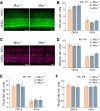
Mitochondrial Ca2+ regulates a wide range of cell processes, including morphogenesis, metabolism, excitotoxicity, and survival. In cochlear hair cells, the activation of mechano-electrical transduction and voltage-gated Ca2+ channels result in a large influx of Ca2+. The intracellular rise in Ca2+ is partly balanced by the mitochondria which rapidly uptakes Ca2+ via a highly selective channel comprised of the main pore-forming subunit, the mitochondrial Ca2+ uniporter (MCU), and associated regulatory proteins. MCU thus contributes to Ca2+ buffering, ensuring cytosolic homeostasis, and is posited to have a critical role in hair cell function and hearing. To test this hypothesis, Ca2+ homeostasis in hair cells and cochlear function were investigated in FVB/NJ mice carrying the knockout allele of Mcu (Mcu+/- or Mcu-/-). The Mcu knockout allele, which originated in C57BL/6 strain cosegregated along with Cdh23ahl allele to the FVB/NJ strain, due to the close proximity of these genes. Neither Mcu+/- nor Mcu-/- genotypes affected cochlear development, morphology, or Ca2+ homeostasis of auditory hair cells in the first two postnatal weeks. However, Mcu-/- mice displayed high-frequency hearing impairment as early as 3 weeks postnatal, which then progressed to profound hearing loss at all frequencies in about 6 months. In Mcu+/- mice, significantly elevated ABR thresholds were observed at 6 months and 9 months of age only at 32 kHz frequency. In three-month-old Mcu-/- mice, up to 18% of the outer hair cells and occasionally some inner hair cells were missing in the mid-cochlear region. In conclusion, mitochondrial Ca2+ uniporter is not required for the development of cochlea in mice, but is essential for hearing and hair cell preservation in congenic FVB/NJ mice.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice.Hear Res. 2012 Jan;283(1-2):80-8. doi: 10.1016/j.heares.2011.11.007. Epub 2011 Nov 22. Hear Res. 2012. PMID: 22138310 Free PMC article.
-
MCU Interacts with Miro1 to Modulate Mitochondrial Functions in Neurons.J Neurosci. 2018 May 16;38(20):4666-4677. doi: 10.1523/JNEUROSCI.0504-18.2018. Epub 2018 Apr 23. J Neurosci. 2018. PMID: 29686046 Free PMC article.
-
The effect of mitochondrial calcium uniporter and cyclophilin D knockout on resistance of brain mitochondria to Ca2+-induced damage.J Biol Chem. 2021 Jan-Jun;296:100669. doi: 10.1016/j.jbc.2021.100669. Epub 2021 Apr 16. J Biol Chem. 2021. PMID: 33864812 Free PMC article.
-
Mitochondrial VDAC, the Na+/Ca2+ Exchanger, and the Ca2+ Uniporter in Ca2+ Dynamics and Signaling.Adv Exp Med Biol. 2017;981:323-347. doi: 10.1007/978-3-319-55858-5_13. Adv Exp Med Biol. 2017. PMID: 29594867 Review.
-
Tonotopy in calcium homeostasis and vulnerability of cochlear hair cells.Hear Res. 2019 May;376:11-21. doi: 10.1016/j.heares.2018.11.002. Epub 2018 Nov 16. Hear Res. 2019. PMID: 30473131 Free PMC article. Review.
Cited by
-
Impacts of impaired mitochondrial dynamics in hearing loss: Potential therapeutic targets.Front Neurosci. 2022 Oct 5;16:998507. doi: 10.3389/fnins.2022.998507. eCollection 2022. Front Neurosci. 2022. PMID: 36278017 Free PMC article. Review.
-
Absence of oncomodulin increases susceptibility to noise-induced outer hair cell death and alters mitochondrial morphology.Front Neurol. 2024 Oct 23;15:1435749. doi: 10.3389/fneur.2024.1435749. eCollection 2024. Front Neurol. 2024. PMID: 39507624 Free PMC article.
-
Mitochondrial calcium cycling in neuronal function and neurodegeneration.Front Cell Dev Biol. 2023 Jan 24;11:1094356. doi: 10.3389/fcell.2023.1094356. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36760367 Free PMC article. Review.
-
Mitochondrial form and function in hair cells.Hear Res. 2023 Feb;428:108660. doi: 10.1016/j.heares.2022.108660. Epub 2022 Nov 25. Hear Res. 2023. PMID: 36525891 Free PMC article. Review.
-
Cytosolic calcium regulates hepatic mitochondrial oxidation, intrahepatic lipolysis, and gluconeogenesis via CAMKII activation.Cell Metab. 2024 Oct 1;36(10):2329-2340.e4. doi: 10.1016/j.cmet.2024.07.016. Epub 2024 Aug 16. Cell Metab. 2024. PMID: 39153480
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

