Umbilical mesenchymal stem cell-derived extracellular vesicles as enzyme delivery vehicle to treat Morquio A fibroblasts
- PMID: 33957983
- PMCID: PMC8101245
- DOI: 10.1186/s13287-021-02355-0
Umbilical mesenchymal stem cell-derived extracellular vesicles as enzyme delivery vehicle to treat Morquio A fibroblasts
Abstract
Background: Mucopolysaccharidosis IVA (Morquio A syndrome) is a lysosomal storage disease caused by the deficiency of enzyme N-acetylgalactosamine-6-sulfate sulfatase (GALNS), which results in the accumulation of the glycosaminoglycans (GAGs), keratan sulfate, and chondroitin-6-sulfate in the lysosomes of all tissues causing systemic dysfunction. Current treatments include enzyme replacement therapy (ERT) which can treat only certain aspects of the disease such as endurance-related biological endpoints. A key challenge in ERT is ineffective enzyme uptake in avascular tissues, which makes the treatment of the corneal, cartilage, and heart valvular tissue difficult. The aim of this study was to culture human umbilical mesenchymal stem cells (UMSC), demonstrate presence of GALNS enzyme activity within the extracellular vesicles (EVs) derived from these UMSC, and study how these secreted EVs are taken up by GALNS-deficient cells and used by the deficient cell's lysosomes.
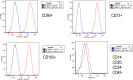
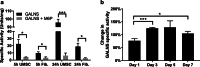
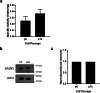
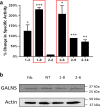
Methods: We obtained and cultured UMSC from the umbilical cord tissue from anonymous donors from the Saint Louis Cord Blood Bank. We characterized UMSC cell surface markers to confirm phenotype by cell sorting analyses. In addition, we confirmed that UMSC secrete GALNS enzyme creating conditioned media for co-culture experiments with GALNS deficient cells. Lastly, we isolated EVs derived from UMSC by ultracentrifugation to confirm source of GALNS enzyme.
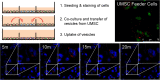
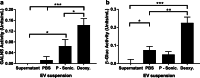
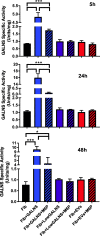
Results: Co-culture and confocal microscopy experiments indicated that the lysosomal content from UMSC migrated to deficient cells as evidenced by the peak signal intensity occurring at 15 min. EVs released by UMSC were characterized indicating that the EVs contained the active GALNS enzyme. Uptake of GALNS within EVs by deficient fibroblasts was not affected by mannose-6-phosphate (M6P) inhibition, suggesting that EV uptake by these fibroblasts is gradual and might be mediated by a different means than the M6P receptor.
Conclusions: UMSC can deliver EVs containing functional GALNS enzyme to deficient cells. This enzyme delivery method, which was unaffected by M6P inhibition, can function as a novel technique for reducing GAG accumulation in cells in avascular tissues, thereby providing a potential treatment option for Morquio A syndrome.
Keywords: Extracellular vesicles; Morquio A; Mucopolysaccharidosis IVA; Umbilical mesenchymal stem cell.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Molecular testing of 163 patients with Morquio A (Mucopolysaccharidosis IVA) identifies 39 novel GALNS mutations.Mol Genet Metab. 2014 Jun;112(2):160-70. doi: 10.1016/j.ymgme.2014.03.004. Epub 2014 Mar 20. Mol Genet Metab. 2014. PMID: 24726177 Free PMC article.
-
Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA.Mol Genet Metab. 2013 Sep-Oct;110(1-2):54-64. doi: 10.1016/j.ymgme.2013.04.002. Epub 2013 Apr 10. Mol Genet Metab. 2013. PMID: 23665161 Free PMC article. Review.
-
Four novel mutations in the N-acetylgalactosamine-6-sulfate sulfatase gene among Egyptian patients with Morquio A disease.Gene. 2017 Feb 5;600:48-54. doi: 10.1016/j.gene.2016.11.002. Epub 2016 Nov 5. Gene. 2017. PMID: 27825773
-
Enzyme replacement in a human model of mucopolysaccharidosis IVA in vitro and its biodistribution in the cartilage of wild type mice.PLoS One. 2010 Aug 16;5(8):e12194. doi: 10.1371/journal.pone.0012194. PLoS One. 2010. PMID: 20808938 Free PMC article.
-
[Mucopolysaccharidosis IVA (Morquio A syndrome): clinical, biological and therapeutic aspects].Ann Biol Clin (Paris). 2007 Jan-Feb;65(1):5-11. Ann Biol Clin (Paris). 2007. PMID: 17264033 Review. French.
Cited by
-
Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review.Pharmaceutics. 2023 Apr 6;15(4):1167. doi: 10.3390/pharmaceutics15041167. Pharmaceutics. 2023. PMID: 37111652 Free PMC article. Review.
-
Cellular microenvironment: a key for tuning mesenchymal stem cell senescence.Front Cell Dev Biol. 2023 Dec 4;11:1323678. doi: 10.3389/fcell.2023.1323678. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38111850 Free PMC article. Review.
-
Evidence of Lysosomal β-Hexosaminidase Enzymatic Activity Associated with Extracellular Vesicles: Potential Applications for the Correction of Sandhoff Disease.J Funct Biomater. 2024 Jun 4;15(6):153. doi: 10.3390/jfb15060153. J Funct Biomater. 2024. PMID: 38921527 Free PMC article.
References
-
- Wang RY, Rudser KD, Dengel DR, Evanoff N, Steinberger J, Movsesyan N, Garrett R, Christensen K, Boylan D, Braddock SR, Shinawi M, Gan Q, Montaño AM. Abnormally increased carotid intima media-thickness and elasticity in patients with Morquio A disease. Orphanet J Rare Dis. 2020;15(1):73. doi: 10.1186/s13023-020-1331-y. - DOI - PMC - PubMed
-
- Tomatsu S, Mackenzie WG, Theroux MC, Mason RW, Thacker MM, Shaffer TH, Montaño AM, Rowan D, Sly W, Alméciga-Díaz CJ, Barrera LA, Chinen Y, Yasuda E, Ruhnke K, Suzuki Y, Orii T. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res Rep Endocr Disord. 2012;2012(2):65–77. doi: 10.2147/RRED.S37278. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

