Rapamycin protects against aristolochic acid nephropathy in mice by potentiating mammalian target of rapamycin‑mediated autophagy
- PMID: 33955513
- PMCID: PMC8127069
- DOI: 10.3892/mmr.2021.12134
Rapamycin protects against aristolochic acid nephropathy in mice by potentiating mammalian target of rapamycin‑mediated autophagy
Abstract
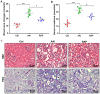
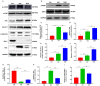
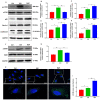
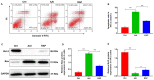
Autophagy serves a crucial role in the etiology of kidney diseases, including drug‑induced renal impairment, inherited kidney disease, diabetic nephropathy and aristolochic acid nephropathy (AAN) and is, therefore, a potential target for treatment. We previously demonstrated that rapamycin could attenuate AAN in mice; however, the underlying mechanism remains to be elucidated. Therefore, whether the renal protective effect of rapamycin (an autophagy activator) is related to autophagy in aristolochic acid (AA)‑treated mice was of particular interest. The pathophysiological roles of rapamycin were investigated in AA‑induced nephrotoxicity in mice and the mechanisms of rapamycin action were explored by evaluating the modulation of autophagy in rapamycin‑treated mice and cultured renal tubular epithelial cells. Supplementation with rapamycin reversed AA‑induced kidney injury in mice and improved AA‑induced autophagy damage in vivo and in vitro. Mechanistically, rapamycin inhibited the renal expression of phosphorylated (p‑)mammalian target of rapamycin (mTOR) and p‑ribosomal S6 protein kinase 1, which in turn activated renal autophagy and decreased apoptosis, probably by removing AA‑elicited damaged mitochondria and misfolded proteins. The findings of the present study demonstrated that rapamycin protects against AA‑induced nephropathy by activating the mTOR‑autophagy axis and suggested that rapamycin may be a promising pharmacological target for the treatment of AAN.
Keywords: apoptosis; aristolochic acid nephropathy; autophagy; mTOR; rapamycin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Renal Protective Effects of 17β-Estradiol on Mice with Acute Aristolochic Acid Nephropathy.Molecules. 2016 Oct 18;21(10):1391. doi: 10.3390/molecules21101391. Molecules. 2016. PMID: 27763560 Free PMC article.
-
Rapamycin-induced autophagy protects proximal tubular renal cells against proteinuric damage through the transcriptional activation of the nerve growth factor receptor NGFR.Autophagy. 2018;14(6):1028-1042. doi: 10.1080/15548627.2018.1448740. Epub 2018 May 11. Autophagy. 2018. PMID: 29749806 Free PMC article.
-
The renoprotective effect of diosgenin on aristolochic acid I-induced renal injury in rats: impact on apoptosis, mitochondrial dynamics and autophagy.Food Funct. 2020 Sep 23;11(9):7456-7467. doi: 10.1039/d0fo00401d. Food Funct. 2020. PMID: 32789347
-
[Aristolochic acid nephropathy: an issue worth more attention].Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013 Apr;33(4):554-8. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013. PMID: 23841283 Review. Chinese.
-
The role of biotransformation enzymes in the development of renal injury and urothelial cancer caused by aristolochic acid: urgent questions and difficult answers.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009 Mar;153(1):5-11. doi: 10.5507/bp.2009.001. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009. PMID: 19365519 Review.
Cited by
-
The Ginsenoside Rg1 Rescues Mitochondrial Disorders in Aristolochic Acid-Induced Nephropathic Mice.Life (Basel). 2021 Sep 27;11(10):1018. doi: 10.3390/life11101018. Life (Basel). 2021. PMID: 34685389 Free PMC article.
-
High Level of Aristolochic Acid Detected With a Unique Genomic Landscape Predicts Early UTUC Onset After Renal Transplantation in Taiwan.Front Oncol. 2022 Jan 6;11:828314. doi: 10.3389/fonc.2021.828314. eCollection 2021. Front Oncol. 2022. PMID: 35071023 Free PMC article.
-
Metabolism at the crossroads of inflammation and fibrosis in chronic kidney disease.Nat Rev Nephrol. 2025 Jan;21(1):39-56. doi: 10.1038/s41581-024-00889-z. Epub 2024 Sep 17. Nat Rev Nephrol. 2025. PMID: 39289568 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

