The human anti-CD40 agonist antibody mitazalimab (ADC-1013; JNJ-64457107) activates antigen-presenting cells, improves expansion of antigen-specific T cells, and enhances anti-tumor efficacy of a model cancer vaccine in vivo
- PMID: 33948686
- PMCID: PMC8571159
- DOI: 10.1007/s00262-021-02932-5
The human anti-CD40 agonist antibody mitazalimab (ADC-1013; JNJ-64457107) activates antigen-presenting cells, improves expansion of antigen-specific T cells, and enhances anti-tumor efficacy of a model cancer vaccine in vivo
Abstract
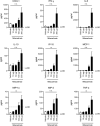
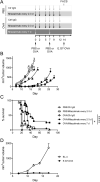
Non-responders to checkpoint inhibitors generally have low tumor T cell infiltration and could benefit from immunotherapy that activates dendritic cells, with priming of tumor-reactive T cells as a result. Such therapies may be augmented by providing tumor antigen in the form of cancer vaccines. Our aim was to study the effects of mitazalimab (ADC-1013; JNJ-64457107), a human anti-CD40 agonist IgG1 antibody, on activation of antigen-presenting cells, and how this influences the priming and anti-tumor potential of antigen-specific T cells, in mice transgenic for human CD40. Mitazalimab activated splenic CD11c+ MHCII+ dendritic cells and CD19+ MHCII+ B cells within 6 h, with a return to baseline within 1 week. This was associated with a dose-dependent release of proinflammatory cytokines in the blood, including IP-10, MIP-1α and TNF-α. Mitazalimab administered at different dose regimens with ovalbumin protein showed that repeated dosing expanded ovalbumin peptide (SIINFEKL)-specific CD8+ T cells and increased the frequency of activated ICOS+ T cells and CD44hi CD62L- effector memory T cells in the spleen. Mitazalimab prolonged survival of mice bearing MB49 bladder carcinoma tumors and increased the frequency of activated granzyme B+ CD8+ T cells in the tumor. In the ovalbumin-transfected tumor E.G7-OVA lymphoma, mitazalimab administered with either ovalbumin protein or SIINFEKL peptide prolonged the survival of E.G7-OVA tumor-bearing mice, as prophylactic and therapeutic treatment. Thus, mitazalimab activates antigen-presenting cells, which improves expansion and activation of antigen-specific T cells and enhances the anti-tumor efficacy of a model cancer vaccine.
Keywords: CD40 agonist antibody; Cancer immunotherapy; Cancer vaccine; Dendritic cell activation.
© 2021. The Author(s).
Conflict of interest statement
All authors are current employees of, and hold stocks or stock options in, Alligator Bioscience AB.
Figures




Similar articles
-
CD40-activated B cells induce anti-tumor immunity in vivo.Oncotarget. 2017 Apr 25;8(17):27740-27753. doi: 10.18632/oncotarget.7720. Oncotarget. 2017. PMID: 26934557 Free PMC article.
-
Anti-CD40 Antibody Fused to CD40 Ligand Is a Superagonist Platform for Adjuvant Intrinsic DC-Targeting Vaccines.Front Immunol. 2022 Jan 13;12:786144. doi: 10.3389/fimmu.2021.786144. eCollection 2021. Front Immunol. 2022. PMID: 35095862 Free PMC article.
-
Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells.J Immunol. 2005 Aug 1;175(3):1424-32. doi: 10.4049/jimmunol.175.3.1424. J Immunol. 2005. PMID: 16034078
-
Next-generation CD40 agonists for cancer immunotherapy.Expert Opin Biol Ther. 2024 May;24(5):351-363. doi: 10.1080/14712598.2024.2357714. Epub 2024 May 23. Expert Opin Biol Ther. 2024. PMID: 38764393 Review.
-
Antigen-Specific Stimulation of CD8+ T-cells by Murine Bone Marrow-Derived Dendritic Cells: Version 1.2019 Feb. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-35. 2019 Feb. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-35. PMID: 39013000 Free Books & Documents. Review.
Cited by
-
The Heterogeneity of the Tumor Microenvironment as Essential Determinant of Development, Progression and Therapy Response of Pancreatic Cancer.Cancers (Basel). 2021 Sep 30;13(19):4932. doi: 10.3390/cancers13194932. Cancers (Basel). 2021. PMID: 34638420 Free PMC article. Review.
-
Early Pharmacodynamic Changes Measured Using RNA Sequencing of Peripheral Blood from Patients in a Phase I Study with Mitazalimab, a Potent CD40 Agonistic Monoclonal Antibody.Cells. 2023 Sep 27;12(19):2365. doi: 10.3390/cells12192365. Cells. 2023. PMID: 37830579 Free PMC article. Clinical Trial.
-
Identification and Validation of Genomic Subtypes and a Prognostic Model Based on Antigen-Presenting Cells and Tumor Microenvironment Infiltration Characteristics in Hepatocellular Carcinoma.Front Oncol. 2022 Jun 3;12:887008. doi: 10.3389/fonc.2022.887008. eCollection 2022. Front Oncol. 2022. PMID: 35720008 Free PMC article.
-
Fc-based Duokines: dual-acting costimulatory molecules comprising TNFSF ligands in the single-chain format fused to a heterodimerizing Fc (scDk-Fc).Oncoimmunology. 2022 Jan 20;11(1):2028961. doi: 10.1080/2162402X.2022.2028961. eCollection 2022. Oncoimmunology. 2022. PMID: 35083097 Free PMC article.
-
XFab-α4-1BB/CD40L fusion protein activates dendritic cells, improves expansion of antigen-specific T cells, and exhibits antitumour efficacy in multiple solid tumour models.Cancer Immunol Immunother. 2023 Dec;72(12):4015-4030. doi: 10.1007/s00262-023-03535-y. Epub 2023 Oct 21. Cancer Immunol Immunother. 2023. PMID: 37863852 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

