Human Cytomegalovirus Uses a Host Stress Response To Balance the Elongation of Saturated/Monounsaturated and Polyunsaturated Very-Long-Chain Fatty Acids
- PMID: 33947752
- PMCID: PMC8262922
- DOI: 10.1128/mBio.00167-21
Human Cytomegalovirus Uses a Host Stress Response To Balance the Elongation of Saturated/Monounsaturated and Polyunsaturated Very-Long-Chain Fatty Acids
Abstract
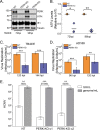
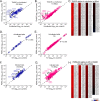
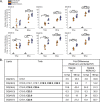
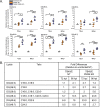
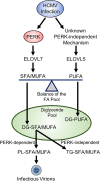
Stress and virus infection regulate lipid metabolism. Human cytomegalovirus (HCMV) infection induces fatty acid (FA) elongation and increases the abundance of lipids with very-long-chain FA (VLCFA) tails. While reprogramming of metabolism can be stress related, the role of stress in HCMV reprogramming of lipid metabolism is poorly understood. In this study, we engineered cells to knock out protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) in the ER stress pathway and measured lipid changes using lipidomics to determine if PERK is needed for lipid changes associated with HCMV infection. In HCMV-infected cells, PERK promotes increases in the levels of phospholipids with saturated FA (SFA) and monounsaturated FA (MUFA) VLCFA tails. Further, PERK enhances FA elongase 7 (ELOVL7) protein levels, which elongates SFA and MUFA VLCFAs. Additionally, we found that increases in the elongation of polyunsaturated fatty acids (PUFAs) associated with HCMV infection were independent of PERK and that lipids with PUFA tails accumulated in HCMV-infected PERK knockout cells. Additionally, the protein levels of ELOVL5, which elongates PUFAs, are increased by HCMV infection through a PERK-independent mechanism. These observations show that PERK differentially regulates ELOVL7 and ELOVL5, creating a balance between the synthesis of lipids with SFA/MUFA tails and PUFA tails. Additionally, we found that PERK was necessary for virus replication and the infectivity of released viral progeny. Overall, our findings indicate that PERK-and, more broadly, ER stress-may be necessary for the membrane biogenesis needed to generate infectious HCMV virions.IMPORTANCE HCMV is a common herpesvirus that establishes lifelong persistent infections. While infection is asymptomatic in most people, HCMV causes life-threatening illnesses in immunocompromised people, including transplant recipients and cancer patients. Additionally, HCMV infection is a leading cause of congenital disabilities. HCMV replication relies on lipid synthesis. Here, we demonstrated that the ER stress mediator PERK controls FA elongation and the cellular abundance of several types of lipids following HCMV infection. Specifically, PERK promotes FA elongase 7 synthesis and phospholipids with saturated/monounsaturated very-long-chain FA tails. Overall, our study shows that PERK is an essential host factor that supports HCMV replication and promotes lipidome changes caused by HCMV infection.
Keywords: ER stress; PERK; fatty acid elongases; herpesviruses; human cytomegalovirus; lipidomics; lipids; very-long-chain fatty acids.
Copyright © 2021 Xi et al.
Figures





Similar articles
-
Human Cytomegalovirus pUL37x1 Is Important for Remodeling of Host Lipid Metabolism.J Virol. 2019 Oct 15;93(21):e00843-19. doi: 10.1128/JVI.00843-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31391267 Free PMC article.
-
Fatty acid elongase 7 catalyzes lipidome remodeling essential for human cytomegalovirus replication.Cell Rep. 2015 Mar 3;10(8):1375-85. doi: 10.1016/j.celrep.2015.02.003. Epub 2015 Feb 26. Cell Rep. 2015. PMID: 25732827 Free PMC article.
-
PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection.PLoS Pathog. 2013;9(4):e1003266. doi: 10.1371/journal.ppat.1003266. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592989 Free PMC article.
-
Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease.Lipids. 2016 May;51(5):507-17. doi: 10.1007/s11745-015-4113-x. Epub 2015 Dec 30. Lipids. 2016. PMID: 26719191 Review.
-
Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans.Eur J Nutr. 2014 Apr;53(3):691-710. doi: 10.1007/s00394-013-0638-z. Epub 2013 Dec 22. Eur J Nutr. 2014. PMID: 24363161 Review.
Cited by
-
Cytomegalovirus drives Vδ1+ γδ T cell expansion and clonality in common variable immunodeficiency.Nat Commun. 2024 May 20;15(1):4286. doi: 10.1038/s41467-024-48527-3. Nat Commun. 2024. PMID: 38769332 Free PMC article.
-
Acute murine cytomegalovirus infection boosts cell-type specific response and lipid metabolism changes in the liver of infant mice.Front Immunol. 2023 Aug 10;14:1169869. doi: 10.3389/fimmu.2023.1169869. eCollection 2023. Front Immunol. 2023. PMID: 37638012 Free PMC article.
-
Liver X Receptor-Inducible Host E3 Ligase IDOL Targets a Human Cytomegalovirus Reactivation Determinant.J Virol. 2023 Jul 27;97(7):e0075823. doi: 10.1128/jvi.00758-23. Epub 2023 Jun 20. J Virol. 2023. PMID: 37338407 Free PMC article.
-
Cholesterol Biosynthesis Modulates CSFV Replication.Viruses. 2022 Jun 30;14(7):1450. doi: 10.3390/v14071450. Viruses. 2022. PMID: 35891429 Free PMC article.
-
IFI16 Impacts Metabolic Reprogramming during Human Cytomegalovirus Infection.mBio. 2022 Jun 28;13(3):e0043522. doi: 10.1128/mbio.00435-22. Epub 2022 Apr 14. mBio. 2022. PMID: 35420480 Free PMC article.
References
-
- Rabasa C, Dickson SL. 2016. Impact of stress on metabolism and energy balance. Curr Opin Behav Sci 9:71–77. doi:10.1016/j.cobeha.2016.01.011. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
