Sec17/Sec18 can support membrane fusion without help from completion of SNARE zippering
- PMID: 33944780
- PMCID: PMC8143792
- DOI: 10.7554/eLife.67578
Sec17/Sec18 can support membrane fusion without help from completion of SNARE zippering
Abstract
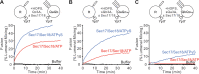
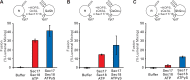
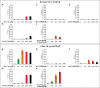
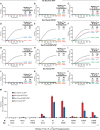
Membrane fusion requires R-, Qa-, Qb-, and Qc-family SNAREs that zipper into RQaQbQc coiled coils, driven by the sequestration of apolar amino acids. Zippering has been thought to provide all the force driving fusion. Sec17/αSNAP can form an oligomeric assembly with SNAREs with the Sec17 C-terminus bound to Sec18/NSF, the central region bound to SNAREs, and a crucial apolar loop near the N-terminus poised to insert into membranes. We now report that Sec17 and Sec18 can drive robust fusion without requiring zippering completion. Zippering-driven fusion is blocked by deleting the C-terminal quarter of any Q-SNARE domain or by replacing the apolar amino acids of the Qa-SNARE that face the center of the 4-SNARE coiled coils with polar residues. These blocks, singly or combined, are bypassed by Sec17 and Sec18, and SNARE-dependent fusion is restored without help from completing zippering.
Keywords: HOPS; S. cerevisiae; SNAREs; Sec17; Sec18; biochemistry; chemical biology; membrane fusion; yeast vacuoles.
© 2021, Song et al.
Conflict of interest statement
HS, TT, AO, WW No competing interests declared, AB Reviewing editor, eLife
Figures

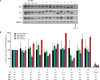
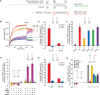
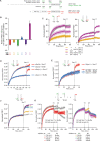
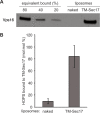






Comment in
-
Molecular machinery turns full circle.Elife. 2021 Jun 17;10:e70298. doi: 10.7554/eLife.70298. Elife. 2021. PMID: 34137372 Free PMC article.
Similar articles
-
Fusion of tethered membranes can be driven by Sec18/NSF and Sec17/αSNAP without HOPS.Elife. 2021 Oct 26;10:e73240. doi: 10.7554/eLife.73240. Elife. 2021. PMID: 34698639 Free PMC article.
-
Sec17/Sec18 act twice, enhancing membrane fusion and then disassembling cis-SNARE complexes.Elife. 2017 Jul 18;6:e26646. doi: 10.7554/eLife.26646. Elife. 2017. PMID: 28718762 Free PMC article.
-
Fusion with wild-type SNARE domains is controlled by juxtamembrane domains, transmembrane anchors, and Sec17.Mol Biol Cell. 2022 May 1;33(5):ar38. doi: 10.1091/mbc.E21-11-0583. Epub 2022 Feb 16. Mol Biol Cell. 2022. PMID: 35171720 Free PMC article.
-
Energetics, kinetics, and pathway of SNARE folding and assembly revealed by optical tweezers.Protein Sci. 2017 Jul;26(7):1252-1265. doi: 10.1002/pro.3116. Epub 2017 Mar 8. Protein Sci. 2017. PMID: 28097727 Free PMC article. Review.
-
A cascade of multiple proteins and lipids catalyzes membrane fusion.Mol Biol Cell. 2017 Mar 15;28(6):707-711. doi: 10.1091/mbc.E16-07-0517. Mol Biol Cell. 2017. PMID: 28292915 Free PMC article. Review.
Cited by
-
Ubiquitination drives COPI priming and Golgi SNARE localization.Elife. 2022 Jul 29;11:e80911. doi: 10.7554/eLife.80911. Elife. 2022. PMID: 35904239 Free PMC article.
-
Sec18 supports membrane fusion by promoting Sec17 membrane association.Mol Biol Cell. 2022 Nov 1;33(13):ar127. doi: 10.1091/mbc.E22-07-0274. Epub 2022 Sep 14. Mol Biol Cell. 2022. PMID: 36103252 Free PMC article.
-
SNARE proteins: zip codes in vesicle targeting?Biochem J. 2022 Feb 11;479(3):273-288. doi: 10.1042/BCJ20210719. Biochem J. 2022. PMID: 35119456 Free PMC article. Review.
-
PI3P regulates multiple stages of membrane fusion.Mol Biol Cell. 2023 Mar 1;34(3):ar17. doi: 10.1091/mbc.E22-10-0486. Epub 2023 Feb 3. Mol Biol Cell. 2023. PMID: 36735517 Free PMC article.
-
Structure of the HOPS tethering complex, a lysosomal membrane fusion machinery.Elife. 2022 Sep 13;11:e80901. doi: 10.7554/eLife.80901. Elife. 2022. PMID: 36098503 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

