Toward Understanding the Molecular Role of SNX27/Retromer in Human Health and Disease
- PMID: 33937239
- PMCID: PMC8083963
- DOI: 10.3389/fcell.2021.642378
Toward Understanding the Molecular Role of SNX27/Retromer in Human Health and Disease
Abstract
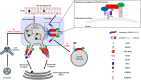
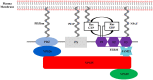
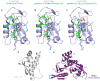
Aberrations in membrane trafficking pathways have profound effects in cellular dynamics of cellular sorting processes and can drive severe physiological outcomes. Sorting nexin 27 (SNX27) is a metazoan-specific sorting nexin protein from the PX-FERM domain family and is required for endosomal recycling of many important transmembrane receptors. Multiple studies have shown SNX27-mediated recycling requires association with retromer, one of the best-known regulators of endosomal trafficking. SNX27/retromer downregulation is strongly linked to Down's Syndrome (DS) via glutamate receptor dysfunction and to Alzheimer's Disease (AD) through increased intracellular production of amyloid peptides from amyloid precursor protein (APP) breakdown. SNX27 is further linked to addiction via its role in potassium channel trafficking, and its over-expression is linked to tumorigenesis, cancer progression, and metastasis. Thus, the correct sorting of multiple receptors by SNX27/retromer is vital for normal cellular function to prevent human diseases. The role of SNX27 in regulating cargo recycling from endosomes to the cell surface is firmly established, but how SNX27 assembles with retromer to generate tubulovesicular carriers remains elusive. Whether SNX27/retromer may be a putative therapeutic target to prevent neurodegenerative disease is now an emerging area of study. This review will provide an update on our molecular understanding of endosomal trafficking events mediated by the SNX27/retromer complex on endosomes.
Keywords: coat proteins; membrane traffic; retromer complex; sorting nexin 27; structural biology.
Copyright © 2021 Chandra, Kendall and Jackson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Retromer Complex and Sorting Nexins in Neurodegenerative Diseases.Front Aging Neurosci. 2018 Mar 26;10:79. doi: 10.3389/fnagi.2018.00079. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29632483 Free PMC article. Review.
-
Actin-Sorting Nexin 27 (SNX27)-Retromer Complex Mediates Rapid Parathyroid Hormone Receptor Recycling.J Biol Chem. 2016 May 20;291(21):10986-1002. doi: 10.1074/jbc.M115.697045. Epub 2016 Mar 23. J Biol Chem. 2016. PMID: 27008860 Free PMC article.
-
Structural basis for coupling of the WASH subunit FAM21 with the endosomal SNX27-Retromer complex.Proc Natl Acad Sci U S A. 2024 Aug 13;121(33):e2405041121. doi: 10.1073/pnas.2405041121. Epub 2024 Aug 8. Proc Natl Acad Sci U S A. 2024. PMID: 39116126
-
Endosomal Sorting Protein SNX27 and Its Emerging Roles in Human Cancers.Cancers (Basel). 2022 Dec 22;15(1):70. doi: 10.3390/cancers15010070. Cancers (Basel). 2022. PMID: 36612066 Free PMC article. Review.
-
SNX27:Retromer:ESCPE-1-mediated early endosomal tubulation impacts cytomegalovirus replication.Front Cell Infect Microbiol. 2024 Sep 18;14:1399761. doi: 10.3389/fcimb.2024.1399761. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39359939 Free PMC article.
Cited by
-
Sorting nexins: role in the regulation of blood pressure.FEBS J. 2023 Feb;290(3):600-619. doi: 10.1111/febs.16305. Epub 2021 Dec 26. FEBS J. 2023. PMID: 34847291 Free PMC article. Review.
-
Assembly and fission of tubular carriers mediating protein sorting in endosomes.Nat Rev Mol Cell Biol. 2024 Oct;25(10):765-783. doi: 10.1038/s41580-024-00746-8. Epub 2024 Jun 17. Nat Rev Mol Cell Biol. 2024. PMID: 38886588 Review.
-
VPS35 or retromer as a potential target for neurodegenerative disorders: barriers to progress.Expert Opin Ther Targets. 2024 Aug;28(8):701-712. doi: 10.1080/14728222.2024.2392700. Epub 2024 Aug 22. Expert Opin Ther Targets. 2024. PMID: 39175128 Review.
-
The cellular pathways that maintain the quality control and transport of diverse potassium channels.Biochim Biophys Acta Gene Regul Mech. 2023 Mar;1866(1):194908. doi: 10.1016/j.bbagrm.2023.194908. Epub 2023 Jan 10. Biochim Biophys Acta Gene Regul Mech. 2023. PMID: 36638864 Free PMC article.
-
The retromer and retriever systems are conserved and differentially expanded in parabasalids.J Cell Sci. 2024 Jul 1;137(13):jcs261949. doi: 10.1242/jcs.261949. Epub 2024 Jul 12. J Cell Sci. 2024. PMID: 38884339 Free PMC article.
References
-
- Balana B., Maslennikov I., Kwiatkowski W., Stern K. M., Bahima L., Choe S., et al. (2011). Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc. Natl. Acad. Sci. U.S.A. 108 5831–5836. 10.1073/pnas.1018645108 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

