Evolution of placentation in cattle and antelopes
- PMID: 33936288
- PMCID: PMC8083812
- DOI: 10.21451/1984-3143-AR2018-00145
Evolution of placentation in cattle and antelopes
Abstract
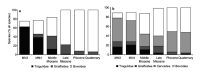
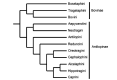
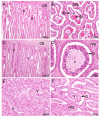
Bovids have enjoyed great evolutionary success as evidenced by the large number of extant species. Several important domestic animals are from this family. They derive from both subfamilies: cattle and their kin belong to Bovinae and sheep and goats to Antilopinae. The premise of this review, therefore, is that evolution of reproduction and placentation is best understood in a context that includes antelope-like bovines and antelopes. Many key features of placentation, including hormone secretion, had evolved before bovids emerged as a distinct group. Variation nevertheless occurs. Most striking is the difference in fusion of the binucleate trophoblast cell with uterine epithelium that yields a transient trinucleate cell in bovines and many antelopes, but a more persistent syncytium in wildebeest, sheep and goat. There is considerable variation in placentome number and villus branching within the placentome. Many antelopes have right-sided implantation in a bicornuate uterus whilst others have a uterus duplex. Finally, there has been continued evolution of placental hormones with tandem duplication of PAG genes in cattle, differences in glycosylation of placental lactogen and the emergence of placental growth hormone in sheep and goats. The selection pressures driving this evolution are unknown though maternal-fetal competition for nutrients is an attractive hypothesis.
Keywords: Antilopinae; Bovidae; Bovinae; binucleate trophoblast cell; chorioallantoic placenta; domestication; fetal membranes; placental hormones; placentome; unilateral implantation.
Copyright © The Author(s). Published by CBRA.
Conflict of interest statement
Conflict of interest statement: The author declares that he has no conflicting interest.
Figures

Similar articles
-
Placentation and hormonal maintenance of pregnancy in the impala (Aepyceros melampus).Placenta. 2020 Jun;95:91-105. doi: 10.1016/j.placenta.2020.04.009. Epub 2020 Apr 23. Placenta. 2020. PMID: 32452408
-
Placentation in the Blue Wildebeest (Connochaetes taurinus).Placenta. 2019 Jul;82:46-56. doi: 10.1016/j.placenta.2019.05.008. Epub 2019 May 29. Placenta. 2019. PMID: 31174626
-
Review: Implantation and placentation in ruminants.Animal. 2023 May;17 Suppl 1:100796. doi: 10.1016/j.animal.2023.100796. Animal. 2023. PMID: 37567669 Review.
-
Immunohistochemical Examination of Trophoblast Syncytialization during Early Placentation in Sheep.Int J Mol Sci. 2019 Sep 13;20(18):4530. doi: 10.3390/ijms20184530. Int J Mol Sci. 2019. PMID: 31540219 Free PMC article.
-
Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses.Physiol Rev. 2012 Oct;92(4):1543-76. doi: 10.1152/physrev.00040.2011. Physiol Rev. 2012. PMID: 23073626 Review.
References
-
- Andresen A. Die Plazentome der Wiederkauer. Morph Jahrbuch Leipzig. 1927;57:410–485.
-
- Assheton R. The morphology of the Ungulate placenta, particularly by the development of that organ in the sheep, and notes upon the placenta of the elephant and Hyrax. Phil Trans R Soc B. 1906;198:143–220.
-
- Belyaev DK. The Wilhelmine E. Key 1978 invitational lecture. Destabilizing selection as a factor in domestication. J Hered. 1979;70(5):301–308. - PubMed
LinkOut - more resources
Full Text Sources
