Targeted Therapy in Cardiovascular Disease: A Precision Therapy Era
- PMID: 33935716
- PMCID: PMC8085499
- DOI: 10.3389/fphar.2021.623674
Targeted Therapy in Cardiovascular Disease: A Precision Therapy Era
Erratum in
-
Corrigendum: Targeted therapy in cardiovascular disease: A precision therapy era.Front Pharmacol. 2023 Feb 3;14:1145460. doi: 10.3389/fphar.2023.1145460. eCollection 2023. Front Pharmacol. 2023. PMID: 36817135 Free PMC article.
Abstract
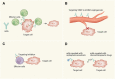
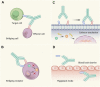
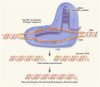
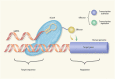
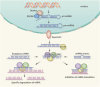
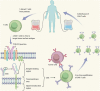
Targeted therapy refers to exploiting the specific therapeutic drugs against the pathogenic molecules (a protein or a gene) or cells. The drug specifically binds to disease-causing molecules or cells without affecting normal tissue, thus enabling personalized and precision treatment. Initially, therapeutic drugs included antibodies and small molecules, (e.g. nucleic acid drugs). With the advancement of the biology technology and immunotherapy, the gene editing and cell editing techniques are utilized for the disease treatment. Currently, targeted therapies applied to treat cardiovascular diseases (CVDs) mainly include protein drugs, gene editing technologies, nucleic acid drugs and cell therapy. Although targeted therapy has demonstrated excellent efficacy in pre-clinical and clinical trials, several limitations need to be recognized and overcome in clinical application, (e.g. off-target events, gene mutations, etc.). This review introduces the mechanisms of different targeted therapies, and mainly describes the targeted therapy applied in the CVDs. Furthermore, we made comparative analysis to clarify the advantages and disadvantages of different targeted therapies. This overview is expected to provide a new concept to the treatment of the CVDs.
Keywords: antibody; cardiovascular disease; cell therapy; gene editing; nucleic acid drugs; targeted therapy.
Copyright © 2021 Xu and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases.Eur Heart J. 2020 Oct 21;41(40):3884-3899. doi: 10.1093/eurheartj/ehaa229. Eur Heart J. 2020. PMID: 32350510
-
Comparative Roles of IL-1, IL-6, IL-10, IL-17, IL-18, 1L-22, IL-33, and IL-37 in Various Cardiovascular Diseases With Potential Insights for Targeted Immunotherapy.Cureus. 2023 Jul 26;15(7):e42494. doi: 10.7759/cureus.42494. eCollection 2023 Jul. Cureus. 2023. PMID: 37637634 Free PMC article. Review.
-
The fourth annual BRDS on genome editing and silencing for precision medicines.Drug Deliv Transl Res. 2018 Feb;8(1):266-272. doi: 10.1007/s13346-017-0457-5. Drug Deliv Transl Res. 2018. PMID: 29209906 Free PMC article.
-
Nanotechnology: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(19):1-43. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074489 Free PMC article.
-
Nucleic acid-based small molecules as targeted transcription therapeutics for immunoregulation.Allergy. 2024 Apr;79(4):843-860. doi: 10.1111/all.15959. Epub 2023 Dec 6. Allergy. 2024. PMID: 38055191 Review.
Cited by
-
A contemporary review of snoRNAs in cardiovascular health: RNA modification and beyond.Mol Ther Nucleic Acids. 2023 Dec 5;35(1):102087. doi: 10.1016/j.omtn.2023.102087. eCollection 2024 Mar 12. Mol Ther Nucleic Acids. 2023. PMID: 38178918 Free PMC article. Review.
-
Extracellular Vesicles in Cardiovascular Diseases: Diagnosis and Therapy.Front Cell Dev Biol. 2022 Jun 1;10:875376. doi: 10.3389/fcell.2022.875376. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35721498 Free PMC article. Review.
-
Nuclear Cardiac Imaging in the Interventional Suite.Curr Cardiol Rep. 2022 Mar;24(3):261-269. doi: 10.1007/s11886-022-01644-1. Epub 2022 Jan 13. Curr Cardiol Rep. 2022. PMID: 35028819 Free PMC article. Review.
-
Myocardial Injury as a Harbinger of Multi-organ Failure in Septic Shock: A Comprehensive Review.Cureus. 2024 Feb 27;16(2):e55021. doi: 10.7759/cureus.55021. eCollection 2024 Feb. Cureus. 2024. PMID: 38550421 Free PMC article. Review.
-
Organic Nanoparticles in Progressing Cardiovascular Disease Treatment and Diagnosis.Polymers (Basel). 2024 May 16;16(10):1421. doi: 10.3390/polym16101421. Polymers (Basel). 2024. PMID: 38794614 Free PMC article. Review.
References
-
- Abbate A., Kontos M. C., Abouzaki N. A., Melchior R. D., Thomas C., Van Tassell B. W., et al. (2015). Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am. J. Cardiol. 115 (3), 288–292. 10.1016/j.amjcard.2014.11.003 - DOI - PubMed
-
- Abifadel M., Rabès, JP, Boileau, C, and Varret, M (2007). [After the LDL receptor and apolipoprotein B, autosomal dominant hypercholesterolemia reveals its third protagonist: PCSK9]. Ann. Endocrinol. (Paris) 68 (2-3), 138–146. 10.1016/j.ando.2007.02.002 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

