UPLC-PDA-ESI-QTOF-MS/MS fingerprint of purified flavonoid enriched fraction of Bryophyllum pinnatum; antioxidant properties, anticholinesterase activity and in silico studies
- PMID: 33930998
- PMCID: PMC8871626
- DOI: 10.1080/13880209.2021.1913189
UPLC-PDA-ESI-QTOF-MS/MS fingerprint of purified flavonoid enriched fraction of Bryophyllum pinnatum; antioxidant properties, anticholinesterase activity and in silico studies
Abstract
Context: Bryophyllum pinnatum (Lam.) Oken (Crassulaceae) is used traditionally to treat many ailments.
Objectives: This study characterizes the constituents of B. pinnatum flavonoid-rich fraction (BPFRF) and investigates their antioxidant and anticholinesterase activity using in vitro and in silico approaches.
Materials and methods: Methanol extract of B. pinnatum leaves was partitioned to yield the ethyl acetate fraction. BPFRF was isolated from the ethyl acetate fraction and purified. The constituent flavonoids were structurally characterized using UPLC-PDA-MS2. Antioxidant activity (DPPH), Fe2+-induced lipid peroxidation (LP) and anticholinesterase activity (Ellman's method) of the BPFRF and standards (ascorbic acid and rivastigmine) across a concentration range of 3.125-100 μg/mL were evaluated in vitro for 4 months. Molecular docking was performed to give insight into the binding potentials of BPFRF constituents against acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE).
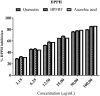
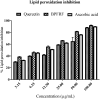
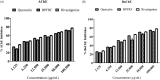
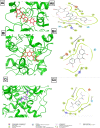
Results: UPLC-PDA-MS2 analysis of BPFRF identified carlinoside, quercetin (most dominant), luteolin, isorhamnetin, luteolin-7-glucoside. Carlinoside was first reported in this plant. BPFRF significantly inhibited DPPH radical (IC50 = 7.382 ± 0.79 µg/mL) and LP (IC50 = 7.182 ± 0.60 µg/mL) better than quercetin and ascorbic acid. Also, BPFRF exhibited potent inhibition against AChE and BuChE with IC50 values of 22.283 ± 0.27 µg/mL and 33.437 ± 1.46 µg/mL, respectively compared to quercetin and rivastigmine. Docking studies revealed that luteolin-7-glucoside, carlinoside and quercetin interact effectively with crucial amino acid residues of AChE and BuChE through hydrogen bonds.
Discussion and conclusions: BPFRF possesses an excellent natural source of cholinesterase inhibitor and antioxidant. The material could be further explored for the potential treatment of oxidative damage and cholinergic dysfunction in Alzheimer's disease.
Keywords: Alzheimer’s diseases; acetylcholinesterase; butyrylcholinesterase; carlinoside; docking studies; lipid peroxidation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Metabolic profiling of Vitex Pubescens Vahl bark via UPLC-ESI-QTOF/MS/MS analysis and evaluation of its antioxidant and acetylcholinesterase inhibitory activities.BMC Complement Med Ther. 2024 Jun 14;24(1):232. doi: 10.1186/s12906-024-04520-3. BMC Complement Med Ther. 2024. PMID: 38877470 Free PMC article.
-
Neuroprotective effect of Bryophyllum pinnatum flavonoids against aluminum chloride-induced neurotoxicity in rats.Toxicol Mech Methods. 2022 May;32(4):243-258. doi: 10.1080/15376516.2021.1995557. Epub 2021 Nov 15. Toxicol Mech Methods. 2022. PMID: 34663170
-
Phenolic profile by HPLC-ESI-MS/MS of six Brazilian Eugenia species and their potential as cholinesterase inhibitors.Nat Prod Res. 2021 Aug;35(15):2608-2611. doi: 10.1080/14786419.2019.1686369. Epub 2019 Nov 4. Nat Prod Res. 2021. PMID: 31680559
-
A Review on the In Vitro Evaluation of the Anticholinesterase Activity Based on Ellman's Method.Mini Rev Med Chem. 2022;22(13):1803-1813. doi: 10.2174/1389557521666211027104638. Mini Rev Med Chem. 2022. PMID: 34711159 Review.
-
Bryophyllum pinnatum (Lam.) Oken: unravelling therapeutic potential and navigating toxicity.Physiol Mol Biol Plants. 2024 Sep;30(9):1413-1427. doi: 10.1007/s12298-024-01509-7. Epub 2024 Sep 11. Physiol Mol Biol Plants. 2024. PMID: 39310702 Review.
Cited by
-
Bioactive Compounds from Kalanchoe Genus Potentially Useful for the Development of New Drugs.Life (Basel). 2023 Feb 26;13(3):646. doi: 10.3390/life13030646. Life (Basel). 2023. PMID: 36983802 Free PMC article. Review.
-
Cleistopholis patens root bark extract exerts cardioprotective effect against doxorubicin-induced myocardial toxicity in rats.Lab Anim Res. 2024 Nov 18;40(1):39. doi: 10.1186/s42826-024-00225-3. Lab Anim Res. 2024. PMID: 39551811 Free PMC article.
-
Computer Aided Drug Design Methodologies with Natural Products in the Drug Research Against Alzheimer's Disease.Curr Neuropharmacol. 2022;20(5):857-885. doi: 10.2174/1570159X19666211005145952. Curr Neuropharmacol. 2022. PMID: 34636299 Free PMC article. Review.
References
-
- Abubakar AA, Oladele HA, Adejumoke A, Kayode FJ.. 2014. Synergistic effect of combined extract of Bryophyllum pinnatum and Aloe barbadensis enhances anti-microbial activity in vitro. Glob Adv Res J Med Med Sci. 3:26–32.
-
- Ademosun AO, Oboh G, Bello F, Ayeni PO.. 2016. Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. J Evid Based Complementary Altern Med. 21(4):NP11–NP17. - PubMed
-
- Ali Reza ASM, Hossain MS, Akhter S, Rahman MR, Nasrin MS, Uddin MJ, Sadik G, Khurshid AHM.. 2018. In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: a study relevant to the treatment of Alzheimer’s disease. BMC Complement Altern Med. 18(1):123–131. - PMC - PubMed
-
- Asaduzzaman M, Uddin M, Kader M, Alam A, Rahman AA, Rashid MK, Kato K, Tanaka T, Takeda M, Sadik G.. 2014. In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aegle marmelos leaf extract: implications for the treatment of Alzheimer’s disease. Psychogeriatrics. 14(1):1–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
