Four distinct trajectories of tau deposition identified in Alzheimer's disease
- PMID: 33927414
- PMCID: PMC8686688
- DOI: 10.1038/s41591-021-01309-6
Four distinct trajectories of tau deposition identified in Alzheimer's disease
Abstract
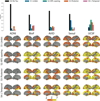
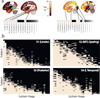
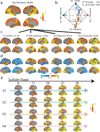
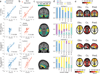
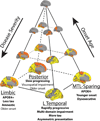
Alzheimer's disease (AD) is characterized by the spread of tau pathology throughout the cerebral cortex. This spreading pattern was thought to be fairly consistent across individuals, although recent work has demonstrated substantial variability in the population with AD. Using tau-positron emission tomography scans from 1,612 individuals, we identified 4 distinct spatiotemporal trajectories of tau pathology, ranging in prevalence from 18 to 33%. We replicated previously described limbic-predominant and medial temporal lobe-sparing patterns, while also discovering posterior and lateral temporal patterns resembling atypical clinical variants of AD. These 'subtypes' were stable during longitudinal follow-up and were replicated in a separate sample using a different radiotracer. The subtypes presented with distinct demographic and cognitive profiles and differing longitudinal outcomes. Additionally, network diffusion models implied that pathology originates and spreads through distinct corticolimbic networks in the different subtypes. Together, our results suggest that variation in tau pathology is common and systematic, perhaps warranting a re-examination of the notion of 'typical AD' and a revisiting of tau pathological staging.
Figures


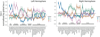
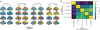
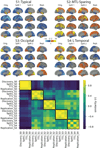
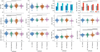
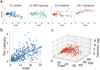
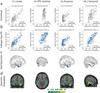


Similar articles
-
Longitudinal neuroimaging biomarkers differ across Alzheimer's disease phenotypes.Brain. 2020 Jul 1;143(7):2281-2294. doi: 10.1093/brain/awaa155. Brain. 2020. PMID: 32572464 Free PMC article.
-
Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer's disease.Alzheimers Dement. 2020 Feb;16(2):335-344. doi: 10.1016/j.jalz.2019.08.201. Epub 2020 Jan 4. Alzheimers Dement. 2020. PMID: 31672482 Free PMC article.
-
Tau Subtypes of Alzheimer's Disease Determined in vivo Using Flortaucipir PET Imaging.J Alzheimers Dis. 2019;71(3):1037-1048. doi: 10.3233/JAD-190264. J Alzheimers Dis. 2019. PMID: 31476153
-
Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative.Alzheimers Dement. 2019 Jan;15(1):106-152. doi: 10.1016/j.jalz.2018.08.005. Epub 2018 Oct 13. Alzheimers Dement. 2019. PMID: 30321505 Review.
-
In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings.Ageing Res Rev. 2017 Jul;36:50-63. doi: 10.1016/j.arr.2017.03.002. Epub 2017 Mar 15. Ageing Res Rev. 2017. PMID: 28315409 Review.
Cited by
-
Physical Exercise and Mechanism Related to Alzheimer's Disease: Is Gut-Brain Axis Involved?Brain Sci. 2024 Sep 27;14(10):974. doi: 10.3390/brainsci14100974. Brain Sci. 2024. PMID: 39451988 Free PMC article. Review.
-
APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer's disease pathology and brain diseases.Mol Neurodegener. 2022 Sep 24;17(1):62. doi: 10.1186/s13024-022-00566-4. Mol Neurodegener. 2022. PMID: 36153580 Free PMC article. Review.
-
Evaluation of Cell-Specific Alterations in Alzheimer's Disease and Relevance of In Vitro Models.Genes (Basel). 2023 Dec 7;14(12):2187. doi: 10.3390/genes14122187. Genes (Basel). 2023. PMID: 38137009 Free PMC article.
-
Analyzing heterogeneity in Alzheimer Disease using multimodal normative modeling on imaging-based ATN biomarkers.ArXiv [Preprint]. 2024 Jul 1:arXiv:2404.05748v2. ArXiv. 2024. PMID: 39010871 Free PMC article. Preprint.
-
Association of Seizure Foci and Location of Tau and Amyloid Deposition and Brain Atrophy in Patients With Alzheimer Disease and Seizures.Neurology. 2024 Nov 12;103(9):e209920. doi: 10.1212/WNL.0000000000209920. Epub 2024 Sep 27. Neurology. 2024. PMID: 39331846
References
-
- Hardy J & Selkoe DJ The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science (80-. ). 297, 353–356 (2002). - PubMed
Methods References
-
- Cho H et al. Predicted sequence of cortical tau and amyloid-β deposition in Alzheimer disease spectrum. Neurobiol. Aging 68, 76–84 (2018). - PubMed
-
- Craddock C, Sikka S, Cheung B, Khanuja R, Ghosh SS, Yan C, Li Q, Lurie D, Vogelstein J, Burns R, S. C & Mennes M, Kelly C, Di Martino A, F C. and M. M Towards Automated Analysis of Connectomes: The Configurable Pipeline for the Analysis of Connectomes (C-PAC). Front. Neuroinform. 7, (2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

