iPSCs: A Preclinical Drug Research Tool for Neurological Disorders
- PMID: 33925625
- PMCID: PMC8123805
- DOI: 10.3390/ijms22094596
iPSCs: A Preclinical Drug Research Tool for Neurological Disorders
Abstract
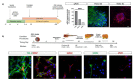
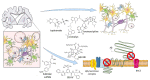
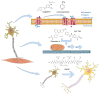
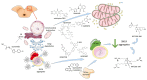
The development and commercialization of new drugs is an articulated, lengthy, and very expensive process that proceeds through several steps, starting from target identification, screening new leading compounds for testing in preclinical studies, and subsequently in clinical trials to reach the final approval for therapeutic use. Preclinical studies are usually performed using both cell cultures and animal models, although they do not completely resume the complexity of human diseases, in particular neurodegenerative conditions. To this regard, stem cells represent a powerful tool in all steps of drug discovery. The recent advancement in induced Pluripotent Stem Cells (iPSCs) technology has opened the possibility to obtain patient-specific disease models for drug screening and development. Here, we report the use of iPSCs as a disease model for drug development in the contest of neurological disorders, including Alzheimer's (AD) and Parkinson's disease (PD), Amyotrophic lateral Sclerosis (ALS), and Fragile X syndrome (FRAX).
Keywords: AD; ALS; FRAX; PD; drug development; iPSCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
-
Advances in Patient-Specific Induced Pluripotent Stem Cells Shed Light on Drug Discovery for Amyotrophic Lateral Sclerosis.Cell Transplant. 2018 Sep;27(9):1301-1312. doi: 10.1177/0963689718785154. Epub 2018 Jul 23. Cell Transplant. 2018. PMID: 30033758 Free PMC article. Review.
-
Neural stem cell-based treatment for neurodegenerative diseases.Neuropathology. 2013 Oct;33(5):491-504. doi: 10.1111/neup.12020. Epub 2013 Feb 5. Neuropathology. 2013. PMID: 23384285 Review.
-
Human Pluripotent Stem Cells in Neurodegenerative Diseases: Potentials, Advances and Limitations.Curr Stem Cell Res Ther. 2020;15(2):102-110. doi: 10.2174/1574888X14666190823142911. Curr Stem Cell Res Ther. 2020. PMID: 31441732 Review.
-
Induced Pluripotent Stem Cell (iPSC)-Based Neurodegenerative Disease Models for Phenotype Recapitulation and Drug Screening.Molecules. 2020 Apr 24;25(8):2000. doi: 10.3390/molecules25082000. Molecules. 2020. PMID: 32344649 Free PMC article. Review.
Cited by
-
Neuroprotective Potential of L-Glutamate Transporters in Human Induced Pluripotent Stem Cell-Derived Neural Cells against Excitotoxicity.Int J Mol Sci. 2023 Aug 9;24(16):12605. doi: 10.3390/ijms241612605. Int J Mol Sci. 2023. PMID: 37628787 Free PMC article.
-
Comprehensive Research on Past and Future Therapeutic Strategies Devoted to Treatment of Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2022 Feb 22;23(5):2400. doi: 10.3390/ijms23052400. Int J Mol Sci. 2022. PMID: 35269543 Free PMC article. Review.
-
The ε-Isozyme of Protein Kinase C (PKCε) Is Impaired in ALS Motor Cortex and Its Pulse Activation by Bryostatin-1 Produces Long Term Survival in Degenerating SOD1-G93A Motor Neuron-like Cells.Int J Mol Sci. 2023 Aug 15;24(16):12825. doi: 10.3390/ijms241612825. Int J Mol Sci. 2023. PMID: 37629005 Free PMC article.
-
Mining human clinical waste as a rich source of stem cells for neural regeneration.iScience. 2024 Jun 19;27(8):110307. doi: 10.1016/j.isci.2024.110307. eCollection 2024 Aug 16. iScience. 2024. PMID: 39156636 Free PMC article. Review.
-
Cellular Models for Primary CoQ Deficiency Pathogenesis Study.Int J Mol Sci. 2021 Sep 22;22(19):10211. doi: 10.3390/ijms221910211. Int J Mol Sci. 2021. PMID: 34638552 Free PMC article. Review.
References
-
- Zang R., Li D., Tang I.-C., Wang J., Yang S.-T. Cell-based assays in high-throughput screening for drug discovery. Int. J. Biotechnol. Wellness Ind. 2012;1:31–51.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

