Microglia and Inhibitory Circuitry in the Medullary Dorsal Horn: Laminar and Time-Dependent Changes in a Trigeminal Model of Neuropathic Pain
- PMID: 33925417
- PMCID: PMC8123867
- DOI: 10.3390/ijms22094564
Microglia and Inhibitory Circuitry in the Medullary Dorsal Horn: Laminar and Time-Dependent Changes in a Trigeminal Model of Neuropathic Pain
Abstract
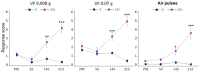
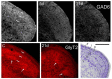
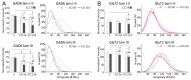
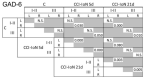
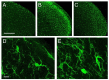
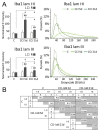
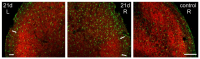
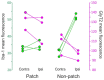
Craniofacial neuropathic pain affects millions of people worldwide and is often difficult to treat. Two key mechanisms underlying this condition are a loss of the negative control exerted by inhibitory interneurons and an early microglial reaction. Basic features of these mechanisms, however, are still poorly understood. Using the chronic constriction injury of the infraorbital nerve (CCI-IoN) model of neuropathic pain in mice, we have examined the changes in the expression of GAD, the synthetic enzyme of GABA, and GlyT2, the membrane transporter of glycine, as well as the microgliosis that occur at early (5 days) and late (21 days) stages post-CCI in the medullary and upper spinal dorsal horn. Our results show that CCI-IoN induces a down-regulation of GAD at both postinjury survival times, uniformly across the superficial laminae. The expression of GlyT2 showed a more discrete and heterogeneous reduction due to the basal presence in lamina III of 'patches' of higher expression, interspersed within a less immunoreactive 'matrix', which showed a more substantial reduction in the expression of GlyT2. These patches coincided with foci lacking any perceptible microglial reaction, which stood out against a more diffuse area of strong microgliosis. These findings may provide clues to better understand the neural mechanisms underlying allodynia in neuropathic pain syndromes.
Keywords: allodynia; chronic pain; glycine transporters; trigeminocervical complex.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Cervical spinal cord injury-induced neuropathic pain in male mice is associated with a persistent pro-inflammatory macrophage/microglial response in the superficial dorsal horn.Exp Neurol. 2021 Sep;343:113757. doi: 10.1016/j.expneurol.2021.113757. Epub 2021 May 12. Exp Neurol. 2021. PMID: 33991526 Free PMC article.
-
Organization of hyperactive microglial cells in trigeminal spinal subnucleus caudalis and upper cervical spinal cord associated with orofacial neuropathic pain.Brain Res. 2012 Apr 27;1451:74-86. doi: 10.1016/j.brainres.2012.02.023. Epub 2012 Feb 17. Brain Res. 2012. PMID: 22459040
-
Arachidonic acid containing phosphatidylcholine increases due to microglial activation in ipsilateral spinal dorsal horn following spared sciatic nerve injury.PLoS One. 2017 May 24;12(5):e0177595. doi: 10.1371/journal.pone.0177595. eCollection 2017. PLoS One. 2017. PMID: 28542572 Free PMC article.
-
TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice.Mol Brain. 2018 Feb 27;11(1):11. doi: 10.1186/s13041-018-0354-y. Mol Brain. 2018. PMID: 29486776 Free PMC article.
-
Glycine transporter inhibitors: A new avenue for managing neuropathic pain.Brain Res Bull. 2019 Oct;152:143-158. doi: 10.1016/j.brainresbull.2019.07.008. Epub 2019 Jul 11. Brain Res Bull. 2019. PMID: 31302238 Review.
Cited by
-
MiR-223-3p alleviates trigeminal neuropathic pain in the male mouse by targeting MKNK2 and MAPK/ERK signaling.Brain Behav. 2022 Jul;12(7):e2634. doi: 10.1002/brb3.2634. Epub 2022 May 24. Brain Behav. 2022. PMID: 35608154 Free PMC article.
References
-
- Baba H., Ji R.R., Kohno T., Moore A.K., Ataka T., Wakai A., Okamoto M., Woolf C.J. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell. Neurosci. 2003;24:818–830. doi: 10.1016/S1044-7431(03)00236-7. - DOI - PubMed

