The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia
- PMID: 33925025
- PMCID: PMC8124869
- DOI: 10.3390/ijms22094657
The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia
Abstract
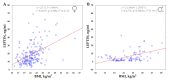
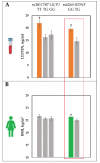
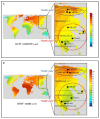
Leptin plays an important role in thermoregulation and is possibly associated with the microevolutionary processes of human adaptation to a cold climate. In this study, based on the Yakut population (n = 281 individuals) living in the coldest region of Siberia (t°minimum -71.2 °C), we analyze the serum leptin levels and data of 14 single nucleotide polymorphisms (SNPs) of 10 genes (UCP1, UCP2, UCP3, FNDC5, PPARGC1A, CIDEA, PTGS2, TRPV1, LEPR, BDNF) that are possibly involved in nonshivering thermogenesis processes. Our results demonstrate that from 14 studied SNPs of 10 genes, 2 SNPs (the TT rs3811787 genotype of the UCP1 gene and the GG rs6265 genotype of the BDNF gene) were associated with the elevated leptin levels in Yakut females (p < 0.05). Furthermore, of these two SNPs, the rs3811787 of the UCP1 gene demonstrated more indications of natural selection for cold climate adaptation. The prevalence gradient of the T-allele (rs3811787) of UCP1 increased from the south to the north across Eurasia, along the shore of the Arctic Ocean. Thereby, our study suggests the potential involvement of the UCP1 gene in the leptin-mediated thermoregulation mechanism, while the distribution of its allelic variants is probably related to human adaptation to a cold climate.
Keywords: Russia; Siberia; UCP1; Yakut population; adaptation; adipose tissue; cold climate; leptin; nonshivering thermogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Relationships between Uncoupling Protein Genes UCP1, UCP2 and UCP3 and Irisin Levels in Residents of the Coldest Region of Siberia.Genes (Basel). 2022 Sep 8;13(9):1612. doi: 10.3390/genes13091612. Genes (Basel). 2022. PMID: 36140780 Free PMC article.
-
Population genetic analysis of the uncoupling proteins supports a role for UCP3 in human cold resistance.Mol Biol Evol. 2011 Jan;28(1):601-14. doi: 10.1093/molbev/msq228. Epub 2010 Aug 28. Mol Biol Evol. 2011. PMID: 20802238 Free PMC article.
-
Cold tolerance of UCP1-ablated mice: a skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up-regulation not associated with increased basal, fatty acid- or ROS-induced uncoupling or enhanced GDP effects.Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):968-80. doi: 10.1016/j.bbabio.2010.02.033. Epub 2010 Mar 19. Biochim Biophys Acta. 2010. PMID: 20227385
-
Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans).Int J Obes (Lond). 2010 Oct;34 Suppl 1:S7-16. doi: 10.1038/ijo.2010.177. Int J Obes (Lond). 2010. PMID: 20935668 Review.
-
UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency.Biochim Biophys Acta. 2001 Mar 1;1504(1):82-106. doi: 10.1016/s0005-2728(00)00247-4. Biochim Biophys Acta. 2001. PMID: 11239487 Review.
Cited by
-
Relationships between Uncoupling Protein Genes UCP1, UCP2 and UCP3 and Irisin Levels in Residents of the Coldest Region of Siberia.Genes (Basel). 2022 Sep 8;13(9):1612. doi: 10.3390/genes13091612. Genes (Basel). 2022. PMID: 36140780 Free PMC article.
-
Epigenetics of the far northern Yakutian population.Clin Epigenetics. 2023 Dec 6;15(1):189. doi: 10.1186/s13148-023-01600-y. Clin Epigenetics. 2023. PMID: 38053163 Free PMC article.
-
A Systematic Review and Meta-Analysis of Free Triiodothyronine (FT3) Levels in Humans Depending on Seasonal Air Temperature Changes: Is the Variation in FT3 Levels Related to Nonshivering Thermogenesis?Int J Mol Sci. 2023 Sep 13;24(18):14052. doi: 10.3390/ijms241814052. Int J Mol Sci. 2023. PMID: 37762355 Free PMC article. Review.
-
NGS Sequencing Reveals New UCP1 Gene Variants Potentially Associated with MetS and/or T2DM Risk in the Polish Population-A Preliminary Study.Genes (Basel). 2023 Mar 24;14(4):789. doi: 10.3390/genes14040789. Genes (Basel). 2023. PMID: 37107547 Free PMC article.
References
-
- Green E.D., Maffei M., Braden V.V., Proenca R., De Silva U., Zhang Y., Chua S.C., Leibel R.L., Weissenbach J., Friedman J.M. The Human Obese (OB) Gene: RNA Expression Pattern and Mapping on the Physical, Cytogenetic, and Genetic Maps of Chromosome 7. Genome Res. 1995;5:5–12. doi: 10.1101/gr.5.1.5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

