Murlentamab, a Low Fucosylated Anti-Müllerian Hormone Type II Receptor (AMHRII) Antibody, Exhibits Anti-Tumor Activity through Tumor-Associated Macrophage Reprogrammation and T Cell Activation
- PMID: 33924378
- PMCID: PMC8070390
- DOI: 10.3390/cancers13081845
Murlentamab, a Low Fucosylated Anti-Müllerian Hormone Type II Receptor (AMHRII) Antibody, Exhibits Anti-Tumor Activity through Tumor-Associated Macrophage Reprogrammation and T Cell Activation
Abstract
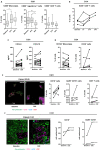
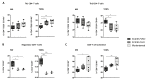
AMHRII, the anti-Müllerian hormone receptor, is selectively expressed in normal sexual organs but is also re-expressed in gynecologic cancers. Hence, we developed murlentamab, a humanized glyco-engineered anti-AMHRII monoclonal antibody currently in clinical trial. Low-fucosylated antibodies are known to increase the antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) potency of effector cells, but some preliminary results suggest a more global murlentamab-dependent activation of the immune system. In this context, we demonstrate here that the murlentamab opsonization of AMHRII-expressing ovarian tumor cells, in the presence of unstimulated- or tumor-associated macrophage (TAM)-like macrophages, significantly promotes macrophage-mediated ADCC and shifts the whole microenvironment towards a pro-inflammatory and anti-tumoral status, thus triggering anti-tumor activity. We also report that murlentamab orients both unstimulated- and TAM-like macrophages to an M1-like phenotype characterized by a strong expression of co-stimulation markers, pro-inflammatory cytokines and chemokines, favoring T cell recruitment and activation. Moreover, we show that murlentamab treatment shifts CD4+ Th1/Th2 balance towards a Th1 response and activates CD8+ T cells. Altogether, these results suggest that murlentamab, through naïve macrophage orientation and TAM reprogrammation, stimulates the anti-tumor adaptive immune response. Those mechanisms might contribute to the sustained clinical benefit observed in advanced cancer patients treated with murlentamab. Finally, the enhanced murlentamab activity in combination with pembrolizumab opens new therapeutic perspectives.
Keywords: adaptive immunity; glyco-engineered antibody; immunotherapy; murlentamab; ovarian cancer; tumor-associated macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The anti-tumor efficacy of 3C23K, a glyco-engineered humanized anti-MISRII antibody, in an ovarian cancer model is mainly mediated by engagement of immune effector cells.Oncotarget. 2017 Jun 6;8(23):37061-37079. doi: 10.18632/oncotarget.15715. Oncotarget. 2017. PMID: 28427157 Free PMC article.
-
The Expression of Anti-Müllerian Hormone Type II Receptor (AMHRII) in Non-Gynecological Solid Tumors Offers Potential for Broad Therapeutic Intervention in Cancer.Biology (Basel). 2021 Apr 7;10(4):305. doi: 10.3390/biology10040305. Biology (Basel). 2021. PMID: 33917111 Free PMC article.
-
The humanized anti-human AMHRII mAb 3C23K exerts an anti-tumor activity against human ovarian cancer through tumor-associated macrophages.Oncotarget. 2017 Oct 7;8(59):99950-99965. doi: 10.18632/oncotarget.21556. eCollection 2017 Nov 21. Oncotarget. 2017. PMID: 29245952 Free PMC article.
-
Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma.Front Immunol. 2018 Nov 5;9:2551. doi: 10.3389/fimmu.2018.02551. eCollection 2018. Front Immunol. 2018. PMID: 30455698 Free PMC article. Review.
-
Macrophages are critical effectors of antibody therapies for cancer.MAbs. 2015;7(2):303-10. doi: 10.1080/19420862.2015.1011450. MAbs. 2015. PMID: 25667985 Free PMC article. Review.
Cited by
-
Anti-Müllerian hormone: biology and role in endocrinology and cancers.Front Endocrinol (Lausanne). 2024 Sep 16;15:1468364. doi: 10.3389/fendo.2024.1468364. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39351532 Free PMC article. Review.
-
Bone morphogenetic protein signaling is a possible therapeutic target in gynecologic cancer.Cancer Sci. 2023 Mar;114(3):722-729. doi: 10.1111/cas.15682. Epub 2022 Dec 16. Cancer Sci. 2023. PMID: 36468782 Free PMC article. Review.
-
Turning adversity into opportunity: Small extracellular vesicles as nanocarriers for tumor-associated macrophages re-education.Bioeng Transl Med. 2022 Jun 9;8(1):e10349. doi: 10.1002/btm2.10349. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684102 Free PMC article. Review.
-
Human macrophage-engineered vesicles for utilization in ovarian cancer treatment.Front Oncol. 2023 Jan 11;12:1042730. doi: 10.3389/fonc.2022.1042730. eCollection 2022. Front Oncol. 2023. PMID: 36713536 Free PMC article.
-
Translational Physiology of Anti-Müllerian Hormone: Clinical Applications in Female Fertility Preservation and Cancer Treatment.Front Endocrinol (Lausanne). 2021 Sep 7;12:689532. doi: 10.3389/fendo.2021.689532. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34557157 Free PMC article. Review.
References
-
- Baarends W.M., van Helmond M.J., Post M., van der Schoot P.J., Hoogerbrugge J.W., de Winter J.P., Uilenbroek J.T., Karels B., Wilming L.G., Meijers J.H. A Novel Member of the Transmembrane Serine/Threonine Kinase Receptor Family Is Specifically Expressed in the Gonads and in Mesenchymal Cells Adjacent to the Müllerian Duct. Dev. Camb. Engl. 1994;120:189–197. - PubMed
-
- Sriraman V., Niu E., Matias J.R., Donahoe P.K., MacLaughlin D.T., Hardy M.P., Lee M.M. Müllerian Inhibiting Substance Inhibits Testosterone Synthesis in Adult Rats. J. Androl. 2001;22:750–758. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

