Polymerized Albumin Receptor of Hepatitis B Virus for Evading the Reticuloendothelial System
- PMID: 33923102
- PMCID: PMC8145202
- DOI: 10.3390/ph14050408
Polymerized Albumin Receptor of Hepatitis B Virus for Evading the Reticuloendothelial System
Abstract
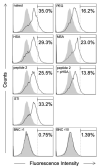
Various strategies, such as optimization of surface chemistry, size, shape, and charge, have been undertaken to develop nanoparticles (NPs) as DDS (drug delivery system) nanocarriers for evading the reticuloendothelial system (RES) in vivo. We previously developed a hollow NP composed of hepatitis B virus (HBV) surface antigen L proteins and lipid bilayers, hereinafter referred to as bio-nanocapsule (BNC), as a nonviral DDS nanocarrier. Such a BNC harbors the HBV-derived human hepatic cell-specific infection mechanism, and intravenously injected BNCs by themselves were shown to avoid clearance by RES-rich organs and accumulate in target tissues. In this study, since the surface modification with albumins is known to prolong the circulation time of nanomedicines, we examined whether the polymerized albumin receptor (PAR) of BNCs contributes to RES evasion in mouse liver. Our results show that NPs conjugated with peptides possessing sufficient PAR activity were captured by Kupffer cells less efficiently in vitro and were able to circulate for a longer period of time in vivo. Comparing with polyethylene glycol, PAR peptides were shown to reduce the recognition by RES to equal content. Taken together, our results strongly suggest that the PAR domain of BNCs, as well as HBV, harbors an innate RES evasion mechanism. Therefore, the surface modification with PAR peptides could be an alternative strategy for improving the pharmacodynamics and pharmacokinetics of forthcoming nanomedicines.
Keywords: albumin; bio-nanocapsule; hepatitis B virus; nanoparticle; polymerized human serum albumin receptor; reticuloendothelial system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Bio-nanocapsules displaying various immunoglobulins as an active targeting-based drug delivery system.Acta Biomater. 2016 Apr 15;35:238-47. doi: 10.1016/j.actbio.2016.02.010. Epub 2016 Feb 10. Acta Biomater. 2016. PMID: 26876802
-
Development of a macrophage-targeting and phagocytosis-inducing bio-nanocapsule-based nanocarrier for drug delivery.Acta Biomater. 2018 Jun;73:412-423. doi: 10.1016/j.actbio.2018.04.023. Epub 2018 Apr 16. Acta Biomater. 2018. PMID: 29673839
-
Development of a virus-mimicking nanocarrier for drug delivery systems: The bio-nanocapsule.Adv Drug Deliv Rev. 2015 Dec 1;95:77-89. doi: 10.1016/j.addr.2015.10.003. Epub 2015 Oct 19. Adv Drug Deliv Rev. 2015. PMID: 26482188 Review.
-
Intracellular trafficking of bio-nanocapsule-liposome complex: Identification of fusogenic activity in the pre-S1 region of hepatitis B virus surface antigen L protein.J Control Release. 2015 Aug 28;212:10-8. doi: 10.1016/j.jconrel.2015.06.012. Epub 2015 Jun 12. J Control Release. 2015. PMID: 26074149
-
[DDS Nanocarriers Mimicking Early Infection Machinery of Viruses].Yakugaku Zasshi. 2020;140(2):147-152. doi: 10.1248/yakushi.19-00187-2. Yakugaku Zasshi. 2020. PMID: 32009036 Review. Japanese.
Cited by
-
Stealth Nanocarriers in Cancer Therapy: a Comprehensive Review of Design, Functionality, and Clinical Applications.AAPS PharmSciTech. 2024 Jun 18;25(6):140. doi: 10.1208/s12249-024-02843-5. AAPS PharmSciTech. 2024. PMID: 38890191 Review.
References
-
- Stolnik S., Illum L., Davis S. Long circulating microparticulate drug carriers. Adv. Drug Deliv. Rev. 1995;16:195–214. doi: 10.1016/0169-409X(95)00025-3. - DOI
-
- Furumoto K., Nagayama S., Ogawara K.-I., Takakura Y., Hashida M., Higaki K., Kimura T. Hepatic uptake of negatively charged particles in rats: Possible involvement of serum proteins in recognition by scavenger receptor. J. Control. Release. 2004;97:133–141. doi: 10.1016/j.jconrel.2004.03.004. - DOI - PubMed
Grants and funding
- 16H06314/Japan Society for the Promotion of Science
- 17J08534/Japan Society for the Promotion of Science
- 17cm0106214h0002/Japan Agency for Medical Research and Development
- 17fk0310105h0001/Japan Agency for Medical Research and Development
- Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials/Ministry of Education, Culture, Sports, Science and Technology of Japan
LinkOut - more resources
Full Text Sources
Miscellaneous

