Role of Virally-Encoded Deubiquitinating Enzymes in Regulation of the Virus Life Cycle
- PMID: 33922750
- PMCID: PMC8123002
- DOI: 10.3390/ijms22094438
Role of Virally-Encoded Deubiquitinating Enzymes in Regulation of the Virus Life Cycle
Abstract
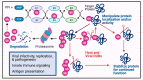
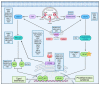
The ubiquitin (Ub) proteasome system (UPS) plays a pivotal role in regulation of numerous cellular processes, including innate and adaptive immune responses that are essential for restriction of the virus life cycle in the infected cells. Deubiquitination by the deubiquitinating enzyme, deubiquitinase (DUB), is a reversible molecular process to remove Ub or Ub chains from the target proteins. Deubiquitination is an integral strategy within the UPS in regulating survival and proliferation of the infecting virus and the virus-invaded cells. Many viruses in the infected cells are reported to encode viral DUB, and these vial DUBs actively disrupt cellular Ub-dependent processes to suppress host antiviral immune response, enhancing virus replication and thus proliferation. This review surveys the types of DUBs encoded by different viruses and their molecular processes for how the infecting viruses take advantage of the DUB system to evade the host immune response and expedite their replication.
Keywords: deubiquitinases (DUBs); ubiquitin proteasome system; ubiquitination; viruses.
Conflict of interest statement
All authors have declared that no competing interests exist.
Figures
Similar articles
-
Structure and Function of Viral Deubiquitinating Enzymes.J Mol Biol. 2017 Nov 10;429(22):3441-3470. doi: 10.1016/j.jmb.2017.06.010. Epub 2017 Jun 16. J Mol Biol. 2017. PMID: 28625850 Free PMC article. Review.
-
Viral deubiquitinases: role in evasion of anti-viral innate immunity.Crit Rev Microbiol. 2018 May;44(3):304-317. doi: 10.1080/1040841X.2017.1368999. Epub 2017 Sep 8. Crit Rev Microbiol. 2018. PMID: 28885059 Review.
-
Human Cytomegalovirus UL48 Deubiquitinase Primarily Targets Innermost Tegument Proteins pp150 and Itself To Regulate Their Stability and Protects Virions from Inclusion of Ubiquitin Conjugates.J Virol. 2021 Nov 9;95(23):e0099121. doi: 10.1128/JVI.00991-21. Epub 2021 Sep 22. J Virol. 2021. PMID: 34549978 Free PMC article.
-
Manipulation of viral infection by deubiquitinating enzymes: new players in host-virus interactions.Future Microbiol. 2016 Oct;11:1435-1446. doi: 10.2217/fmb-2016-0091. Epub 2016 Oct 27. Future Microbiol. 2016. PMID: 27785925 Review.
-
UL36 Encoded by Marek's Disease Virus Exhibits Linkage-Specific Deubiquitinase Activity.Int J Mol Sci. 2020 Mar 5;21(5):1783. doi: 10.3390/ijms21051783. Int J Mol Sci. 2020. PMID: 32150874 Free PMC article.
Cited by
-
Proteome-scale structural prediction of the giant Marseillevirus reveals conserved folds and putative homologs of the hypothetical proteins.Arch Virol. 2024 Oct 16;169(11):222. doi: 10.1007/s00705-024-06155-8. Arch Virol. 2024. PMID: 39414627
-
A Method to Monitor Activity of SARS-CoV-2 Nsp3 from Cells.Methods Mol Biol. 2023;2591:269-282. doi: 10.1007/978-1-0716-2803-4_16. Methods Mol Biol. 2023. PMID: 36350554
-
The Autophagy Receptor SQSTM1/p62 Is a Restriction Factor of HCMV Infection.Viruses. 2024 Sep 10;16(9):1440. doi: 10.3390/v16091440. Viruses. 2024. PMID: 39339916 Free PMC article.
-
Upregulated Proteasome Subunits in COVID-19 Patients: A Link with Hypoxemia, Lymphopenia and Inflammation.Biomolecules. 2022 Mar 13;12(3):442. doi: 10.3390/biom12030442. Biomolecules. 2022. PMID: 35327634 Free PMC article.
-
Factors affecting RIG-I-Like receptors activation - New research direction for viral hemorrhagic fevers.Front Immunol. 2022 Sep 29;13:1010635. doi: 10.3389/fimmu.2022.1010635. eCollection 2022. Front Immunol. 2022. PMID: 36248895 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

