Elevated Interleukin-18 Receptor Accessory Protein Mediates Enhancement in Reactive Oxygen Species Production in Neutrophils of Systemic Lupus Erythematosus Patients
- PMID: 33919154
- PMCID: PMC8143138
- DOI: 10.3390/cells10050964
Elevated Interleukin-18 Receptor Accessory Protein Mediates Enhancement in Reactive Oxygen Species Production in Neutrophils of Systemic Lupus Erythematosus Patients
Abstract
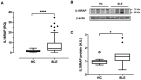
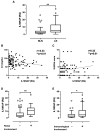
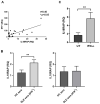
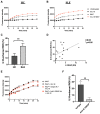
Interleukin-18 receptor accessory protein (IL18RAP) is an indispensable subunit for the IL-18 receptor (IL-18R) complex's ability to mediate high-affinity IL-18 binding and signalling transduction. Interest in IL-18 in systemic lupus erythematosus (SLE) has been mostly focused on its role as a type 1 T helper cell-driving cytokine. The functional significance of IL18RAP in mediating the IL-18-driven response in myeloid cells in SLE remains largely unexplored. This study aimed to investigate the expression and function significance of IL18RAP in neutrophils of SLE patients. By qRT-PCR and Western blot analyses, elevated expressions of IL18RAP mRNA and protein were observed in neutrophils from SLE patients-particularly those with a history of nephritis. IL18RAP expression correlated negatively with complement 3 level and positively with disease activity, with higher expression in patients exhibiting renal and immunological manifestations. The increased IL18RAP expression in SLE neutrophils could be attributed to elevated type I interferon level in sera. Functionally, neutrophils from SLE patients showed higher IL-18-mediated enhancement in reactive oxygen species (ROS) generation, which showed positive correlation with IL18RAP expression and could be neutralized by anti-IL18RAP blocking antibodies. Taken together, our findings suggest that IL-18 could contribute to SLE pathogenesis through mediation of neutrophil dysfunction via the upregulation of IL18RAP expression.
Keywords: SLE; cellular function; interleukin-18 receptor accessory protein; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Neutrophil Extracellular Trap Mitochondrial DNA and Its Autoantibody in Systemic Lupus Erythematosus and a Proof-of-Concept Trial of Metformin.Arthritis Rheumatol. 2015 Dec;67(12):3190-200. doi: 10.1002/art.39296. Arthritis Rheumatol. 2015. PMID: 26245802 Clinical Trial.
-
Differential reactive oxygen species production of neutrophils and their oxidative damage in patients with active and inactive systemic lupus erythematosus.Immunol Lett. 2017 Apr;184:1-6. doi: 10.1016/j.imlet.2017.01.018. Epub 2017 Feb 3. Immunol Lett. 2017. PMID: 28163154
-
Aberrant expression of regulatory cytokine IL-35 in patients with systemic lupus erythematosus.Lupus. 2015 Oct;24(12):1257-66. doi: 10.1177/0961203315585815. Epub 2015 May 11. Lupus. 2015. PMID: 25966926
-
Innate Immune Dysregulation in the Development of Cardiovascular Disease in Lupus.Curr Rheumatol Rep. 2019 Jul 23;21(9):46. doi: 10.1007/s11926-019-0842-9. Curr Rheumatol Rep. 2019. PMID: 31338604 Review.
-
Neutrophils-Important Communicators in Systemic Lupus Erythematosus and Antiphospholipid Syndrome.Front Immunol. 2019 Nov 22;10:2734. doi: 10.3389/fimmu.2019.02734. eCollection 2019. Front Immunol. 2019. PMID: 31824510 Free PMC article. Review.
Cited by
-
Histological differences related to autophagy in the minor salivary gland between primary and secondary types of Sjögren's syndrome.BMC Oral Health. 2024 Sep 16;24(1):1099. doi: 10.1186/s12903-024-04869-4. BMC Oral Health. 2024. PMID: 39285388 Free PMC article.
-
Pangenome obtained by long-read sequencing of 11 genomes reveal hidden functional structural variants in pigs.iScience. 2023 Feb 2;26(3):106119. doi: 10.1016/j.isci.2023.106119. eCollection 2023 Mar 17. iScience. 2023. PMID: 36852268 Free PMC article.
-
Systemic lupus erythematosus: latest insight into etiopathogenesis.Rheumatol Int. 2023 Aug;43(8):1381-1393. doi: 10.1007/s00296-023-05346-x. Epub 2023 May 24. Rheumatol Int. 2023. PMID: 37226016 Free PMC article. Review.
-
Biological and clinical roles of IL-18 in inflammatory diseases.Nat Rev Rheumatol. 2024 Jan;20(1):33-47. doi: 10.1038/s41584-023-01053-w. Epub 2023 Dec 11. Nat Rev Rheumatol. 2024. PMID: 38081945 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

