IL13Rα2 Is Involved in the Progress of Renal Cell Carcinoma through the JAK2/FOXO3 Pathway
- PMID: 33917914
- PMCID: PMC8068290
- DOI: 10.3390/jpm11040284
IL13Rα2 Is Involved in the Progress of Renal Cell Carcinoma through the JAK2/FOXO3 Pathway
Abstract
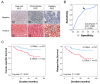
Previously, we reported a close relationship between type II IL4Rα and IL13Rα1 complex and poor outcomes in renal cell carcinoma (RCC). In this study, we investigated the clinicopathologically significant oncogenic role of IL13Rα2, a kind of the independent receptor for IL13, in 229 RCC patients. The high expression of IL13Rα2 was closely related to relapse-free survival in specific cancers in univariate and multivariate analysis. Then, the oncogenic role of IL13Rα2 was evaluated by performing in vitro assays for cell proliferation, cell cycle arrest, and apoptosis in A498, ACHN, Caki1, and Caki2, four kinds of RCC cells after transfection of siRNA against IL13Rα2. Cell proliferation was suppressed, and apoptosis was induced in A498, ACHN, Caki1, and Caki2 cells by knockdown of IL13Rα2. Interestingly, the knockdown of IL13Rα2 decreased the phosphorylation of JAK2 and increased the expression of FOXO3. Furthermore, the knockdown of IL13Rα2 reduced the protein interaction among IL13Rα2, phosphorylated JAK2, and FOXO3. Since phosphorylation of JAK2 was regulated by IL13Rα2, we tried to screen a novel JAK2 inhibitor from the FDA-approved drug library and selected telmisartan, a clinically used medicine against hypertension, as one of the strongest candidates. Telmisartan treatment decreased the cell proliferation rate and increased apoptosis in A498, ACHN, Caki1, and Caki2 cells. Mechanistically, telmisartan treatment decreased the phosphorylation of JAK2 and increased the expression of FOXO3. Taken together, these results suggest that IL13Rα2 regulates the progression of RCC via the JAK2/FOXO3-signaling path pathway, which might be targeted as the novel therapeutic option for RCC patients.
Keywords: FOXO3; IL13Rα2; JAK2; renal cell carcinoma; telmisartan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Interleukin4Rα (IL4Rα) and IL13Rα1 Are Associated with the Progress of Renal Cell Carcinoma through Janus Kinase 2 (JAK2)/Forkhead Box O3 (FOXO3) Pathways.Cancers (Basel). 2019 Sep 18;11(9):1394. doi: 10.3390/cancers11091394. Cancers (Basel). 2019. PMID: 31540495 Free PMC article.
-
IL4Rα and IL13Rα1 Are Involved in the Development of Human Gallbladder Cancer.J Pers Med. 2022 Feb 9;12(2):249. doi: 10.3390/jpm12020249. J Pers Med. 2022. PMID: 35207737 Free PMC article.
-
Expression of interleukin 13 receptor in glioma and renal cell carcinoma: IL13Ralpha2 as a decoy receptor for IL13.Lab Invest. 2001 Sep;81(9):1223-31. doi: 10.1038/labinvest.3780336. Lab Invest. 2001. PMID: 11555670
-
Acetylshikonin, A Novel CYP2J2 Inhibitor, Induces Apoptosis in RCC Cells via FOXO3 Activation and ROS Elevation.Oxid Med Cell Longev. 2022 Mar 9;2022:9139338. doi: 10.1155/2022/9139338. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2024 Jan 9;2024:9846960. doi: 10.1155/2024/9846960. PMID: 35308176 Free PMC article. Retracted.
-
Interleukin-6 induces drug resistance in renal cell carcinoma.Fukushima J Med Sci. 2018 Dec 8;64(3):103-110. doi: 10.5387/fms.2018-15. Epub 2018 Oct 23. Fukushima J Med Sci. 2018. PMID: 30369518 Free PMC article. Review.
Cited by
-
Special Issue "Cancer Biomarker Research and Personalized Medicine".J Pers Med. 2022 Apr 5;12(4):585. doi: 10.3390/jpm12040585. J Pers Med. 2022. PMID: 35455701 Free PMC article.
References
-
- Heng D.Y., Xie W., Regan M.M., Warren M.A., Golshayan A.R., Sahi C., Eigl B.J., Ruether J.D., Cheng T., North S., et al. Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large, Multicenter Study. J. Clin. Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

