Role of Oxidative DNA Damage and Repair in Atrial Fibrillation and Ischemic Heart Disease
- PMID: 33917194
- PMCID: PMC8068079
- DOI: 10.3390/ijms22083838
Role of Oxidative DNA Damage and Repair in Atrial Fibrillation and Ischemic Heart Disease
Abstract
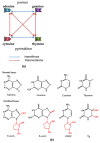
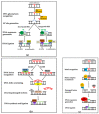
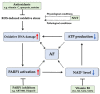
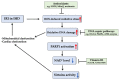
Atrial fibrillation (AF) and ischemic heart disease (IHD) represent the two most common clinical cardiac diseases, characterized by angina, arrhythmia, myocardial damage, and cardiac dysfunction, significantly contributing to cardiovascular morbidity and mortality and posing a heavy socio-economic burden on society worldwide. Current treatments of these two diseases are mainly symptomatic and lack efficacy. There is thus an urgent need to develop novel therapies based on the underlying pathophysiological mechanisms. Emerging evidence indicates that oxidative DNA damage might be a major underlying mechanism that promotes a variety of cardiac diseases, including AF and IHD. Antioxidants, nicotinamide adenine dinucleotide (NAD+) boosters, and enzymes involved in oxidative DNA repair processes have been shown to attenuate oxidative damage to DNA, making them potential therapeutic targets for AF and IHD. In this review, we first summarize the main molecular mechanisms responsible for oxidative DNA damage and repair both in nuclei and mitochondria, then describe the effects of oxidative DNA damage on the development of AF and IHD, and finally discuss potential targets for oxidative DNA repair-based therapeutic approaches for these two cardiac diseases.
Keywords: DNA repair; NAD+; PARP1; antioxidant; atrial fibrillation; cardiac disease; ischemia/reperfusion injury; ischemic heart disease; oxidative DNA damage; vitamin B3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress-mediated myocardial injury phenomenon.Eur J Cardiovasc Prev Rehabil. 2008 Dec;15(6):735-41. doi: 10.1097/HJR.0b013e328317f38a. Eur J Cardiovasc Prev Rehabil. 2008. PMID: 19020458 Review.
-
The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation.Int J Cardiol. 2007 Feb 7;115(2):135-43. doi: 10.1016/j.ijcard.2006.04.026. Epub 2006 Jun 9. Int J Cardiol. 2007. PMID: 16764958 Review.
-
Mechanisms and Treatments of Oxidative Stress in Atrial Fibrillation.Curr Pharm Des. 2018;24(26):3062-3071. doi: 10.2174/1381612824666180903144042. Curr Pharm Des. 2018. PMID: 30179130 Review.
-
DNA damage-induced PARP1 activation confers cardiomyocyte dysfunction through NAD+ depletion in experimental atrial fibrillation.Nat Commun. 2019 Mar 21;10(1):1307. doi: 10.1038/s41467-019-09014-2. Nat Commun. 2019. PMID: 30898999 Free PMC article.
-
Redox control of cardiac remodeling in atrial fibrillation.Biochim Biophys Acta. 2015 Aug;1850(8):1555-65. doi: 10.1016/j.bbagen.2014.12.012. Epub 2014 Dec 13. Biochim Biophys Acta. 2015. PMID: 25513966 Review.
Cited by
-
Disruption of Sarcoplasmic Reticulum-Mitochondrial Contacts Underlies Contractile Dysfunction in Experimental and Human Atrial Fibrillation: A Key Role of Mitofusin 2.J Am Heart Assoc. 2022 Oct 4;11(19):e024478. doi: 10.1161/JAHA.121.024478. Epub 2022 Sep 29. J Am Heart Assoc. 2022. PMID: 36172949 Free PMC article.
-
Inhibition of PLK3 Attenuates Tubular Epithelial Cell Apoptosis after Renal Ischemia-Reperfusion Injury by Blocking the ATM/P53-Mediated DNA Damage Response.Oxid Med Cell Longev. 2022 Jun 24;2022:4201287. doi: 10.1155/2022/4201287. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35783188 Free PMC article.
-
DNA damage and arterial hypertension. A systematic review and meta-analysis.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2024 Mar;168(1):15-24. doi: 10.5507/bp.2023.044. Epub 2023 Oct 30. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2024. PMID: 37916467 Review.
-
XJB-5-131 Is a Mild Uncoupler of Oxidative Phosphorylation.J Huntingtons Dis. 2022;11(2):141-151. doi: 10.3233/JHD-220539. J Huntingtons Dis. 2022. PMID: 35404288 Free PMC article.
-
Association between healthy dietary patterns and markers of oxidative stress in the Sister Study.Eur J Nutr. 2024 Mar;63(2):485-499. doi: 10.1007/s00394-023-03280-z. Epub 2023 Dec 9. Eur J Nutr. 2024. PMID: 38070016
References
-
- Vos T., Allen C., Arora M., Barber R.M., Bhutta Z.A., Brown A., Carter A., Casey D.C., Charlson F.J., Chen A.Z. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

