Identification of Flavonoids as Putative ROS-1 Kinase Inhibitors Using Pharmacophore Modeling for NSCLC Therapeutics
- PMID: 33917039
- PMCID: PMC8067712
- DOI: 10.3390/molecules26082114
Identification of Flavonoids as Putative ROS-1 Kinase Inhibitors Using Pharmacophore Modeling for NSCLC Therapeutics
Abstract
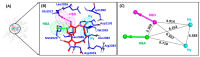
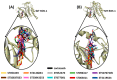
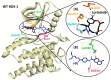
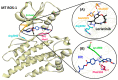
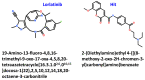
Non-small cell lung cancer (NSCLC) is a lethal non-immunogenic malignancy and proto-oncogene ROS-1 tyrosine kinase is one of its clinically relevant oncogenic markers. The ROS-1 inhibitor, crizotinib, demonstrated resistance due to the Gly2032Arg mutation. To curtail this resistance, researchers developed lorlatinib against the mutated kinase. In the present study, a receptor-ligand pharmacophore model exploiting the key features of lorlatinib binding with ROS-1 was exploited to identify inhibitors against the wild-type (WT) and the mutant (MT) kinase domain. The developed model was utilized to virtually screen the TimTec flavonoids database and the retrieved drug-like hits were subjected for docking with the WT and MT ROS-1 kinase. A total of 10 flavonoids displayed higher docking scores than lorlatinib. Subsequent molecular dynamics simulations of the acquired flavonoids with WT and MT ROS-1 revealed no steric clashes with the Arg2032 (MT ROS-1). The binding free energy calculations computed via molecular mechanics/Poisson-Boltzmann surface area (MM/PBSA) demonstrated one flavonoid (Hit) with better energy than lorlatinib in binding with WT and MT ROS-1. The Hit compound was observed to bind in the ROS-1 selectivity pocket comprised of residues from the β-3 sheet and DFG-motif. The identified Hit from this investigation could act as a potent WT and MT ROS-1 inhibitor.
Keywords: MM/PBSA; NSCLC; ROS-1 kinase; drug resistance; flavonoids; molecular docking; molecular dynamics simulations; structure-based pharmacophore; virtual screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Identification of non-resistant ROS-1 inhibitors using structure based pharmacophore analysis.J Mol Graph Model. 2016 Nov;70:85-93. doi: 10.1016/j.jmgm.2016.09.013. Epub 2016 Sep 29. J Mol Graph Model. 2016. PMID: 27693947
-
Investigation on the binding mechanism of loratinib with the c-ros oncogene 1 (ROS1) receptor tyrosine kinase via molecular dynamics simulation and binding free energy calculations.J Biomol Struct Dyn. 2018 Sep;36(12):3106-3113. doi: 10.1080/07391102.2017.1378127. Epub 2017 Sep 25. J Biomol Struct Dyn. 2018. PMID: 28893136
-
Pharmacophore modeling, multiple docking, and molecular dynamics studies on Wee1 kinase inhibitors.J Biomol Struct Dyn. 2019 Jul;37(10):2703-2715. doi: 10.1080/07391102.2018.1495576. Epub 2018 Dec 24. J Biomol Struct Dyn. 2019. PMID: 30052133
-
Evolution strategy of ROS1 kinase inhibitors for use in cancer therapy.Future Med Chem. 2018 Jul 1;10(14):1705-1720. doi: 10.4155/fmc-2018-0033. Epub 2018 Jul 2. Future Med Chem. 2018. PMID: 29961337 Review.
-
Residue-based pharmacophore approaches to study protein-protein interactions.Curr Opin Struct Biol. 2021 Apr;67:205-211. doi: 10.1016/j.sbi.2020.12.016. Epub 2021 Jan 22. Curr Opin Struct Biol. 2021. PMID: 33486430 Free PMC article. Review.
Cited by
-
Computational Investigation Identified Potential Chemical Scaffolds for Heparanase as Anticancer Therapeutics.Int J Mol Sci. 2021 May 18;22(10):5311. doi: 10.3390/ijms22105311. Int J Mol Sci. 2021. PMID: 34156395 Free PMC article.
-
Flavonoids in Treatment of Chronic Kidney Disease.Molecules. 2022 Apr 6;27(7):2365. doi: 10.3390/molecules27072365. Molecules. 2022. PMID: 35408760 Free PMC article. Review.
-
Identification of CDK7 Inhibitors from Natural Sources Using Pharmacoinformatics and Molecular Dynamics Simulations.Biomedicines. 2021 Sep 10;9(9):1197. doi: 10.3390/biomedicines9091197. Biomedicines. 2021. PMID: 34572383 Free PMC article.
-
Investigation of Marine-Derived Natural Products as Raf Kinase Inhibitory Protein (RKIP)-Binding Ligands.Mar Drugs. 2021 Oct 18;19(10):581. doi: 10.3390/md19100581. Mar Drugs. 2021. PMID: 34677480 Free PMC article.
-
Investigating natural compounds against oncogenic RET tyrosine kinase using pharmacoinformatic approaches for cancer therapeutics.RSC Adv. 2022 Jan 5;12(2):1194-1207. doi: 10.1039/d1ra07328a. eCollection 2021 Dec 22. RSC Adv. 2022. PMID: 35425116 Free PMC article.
References
-
- Rajurkar S., Mambetsariev I., Pharaon R., Leach B., Tan T., Kulkarni P., Salgia R. Non-Small Cell Lung Cancer from Genomics to Therapeutics: A Framework for Community Practice Integration to Arrive at Personalized Therapy Strategies. J. Clin. Med. 2020;9:1870. doi: 10.3390/jcm9061870. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2020R1A6A1A03044344/This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education
- NRF-2018M3A9A70-57263/This research was supported by the Bio & Medical Technology Development Program of the Na-tional Research Foundation (NRF) & funded by the Korean government (MSIT)
LinkOut - more resources
Full Text Sources
Other Literature Sources

