An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity against B Cell Acute Lymphoblastic Leukemia
- PMID: 33915811
- PMCID: PMC8036612
- DOI: 10.3390/cancers13071632
An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity against B Cell Acute Lymphoblastic Leukemia
Abstract
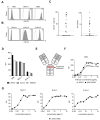
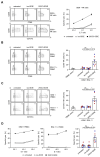
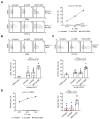
In recent decades, antibody-dependent cellular cytotoxicity (ADCC)-inducing monoclonal antibodies (mAbs) have revolutionized cancer immunotherapy, and Fc engineering strategies have been utilized to further improve efficacy. A promising option is to enhance the affinity of an antibody's Fc-part to the Fc-receptor CD16 by altering the amino acid sequence. Herein, we characterized an S239D/I332E-modified CD133 mAb termed 293C3-SDIE for treatment of B cell acute lymphoblastic leukemia (B-ALL). Flow cytometric analysis revealed CD133 expression on B-ALL cell lines and leukemic cells of 50% (14 of 28) B-ALL patients. 293C3-SDIE potently induced NK cell reactivity against the B-ALL cell lines SEM and RS4;11, as well as leukemic cells of B-ALL patients in a target antigen-dependent manner, as revealed by analysis of NK cell activation, degranulation, and cytotoxicity. Of note, CD133 expression did not correlate with BCR-ABL, CD19, CD20, or CD22, which are presently used as therapeutic targets in B-ALL, which revealed CD133 as an independent target for B-ALL treatment. Increased CD133 expression was also observed in MLL-AF4-rearranged B-ALL, indicating that 293C3-SDIE may constitute a particularly suitable treatment option in this hard-to-treat subpopulation. Taken together, our results identify 293C3-SDIE as a promising therapeutic agent for the treatment of B-ALL.
Keywords: ADCC; B-ALL; CD133; Fc engineering; NK cells; acute lymphoblastic leukemia; immunotherapy; prominin-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia.Leukemia. 2017 Feb;31(2):459-469. doi: 10.1038/leu.2016.194. Epub 2016 Jul 20. Leukemia. 2017. PMID: 27435001
-
An Fc-Optimized CD133 Antibody for Induction of Natural Killer Cell Reactivity against Colorectal Cancer.Cancers (Basel). 2019 Jun 7;11(6):789. doi: 10.3390/cancers11060789. Cancers (Basel). 2019. PMID: 31181683 Free PMC article.
-
Induction of NK Cell Reactivity against B-Cell Acute Lymphoblastic Leukemia by an Fc-Optimized FLT3 Antibody.Cancers (Basel). 2019 Dec 6;11(12):1966. doi: 10.3390/cancers11121966. Cancers (Basel). 2019. PMID: 31817795 Free PMC article.
-
New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies.BioDrugs. 2011 Feb 1;25(1):13-25. doi: 10.2165/11539590-000000000-00000. BioDrugs. 2011. PMID: 21090841 Review.
-
Natural Killer (NK) Cell Education Differentially Influences HIV Antibody-Dependent NK Cell Activation and Antibody-Dependent Cellular Cytotoxicity.Front Immunol. 2017 Aug 24;8:1033. doi: 10.3389/fimmu.2017.01033. eCollection 2017. Front Immunol. 2017. PMID: 28883824 Free PMC article. Review.
Cited by
-
Epithelial-to-mesenchymal transition of circulating tumor cells and CD133 expression on predicting prognosis of thyroid cancer patients.Mol Clin Oncol. 2022 Aug 1;17(3):141. doi: 10.3892/mco.2022.2574. eCollection 2022 Sep. Mol Clin Oncol. 2022. PMID: 36033974 Free PMC article.
-
An Fc-modified monoclonal antibody as novel treatment option for pancreatic cancer.Front Immunol. 2024 Jan 22;15:1343929. doi: 10.3389/fimmu.2024.1343929. eCollection 2024. Front Immunol. 2024. PMID: 38322253 Free PMC article.
-
Leveraging Natural Killer Cell Innate Immunity against Hematologic Malignancies: From Stem Cell Transplant to Adoptive Transfer and Beyond.Int J Mol Sci. 2022 Dec 22;24(1):204. doi: 10.3390/ijms24010204. Int J Mol Sci. 2022. PMID: 36613644 Free PMC article. Review.
-
Principles and current clinical landscape of NK cell engaging bispecific antibody against cancer.Hum Vaccin Immunother. 2023 Aug;19(2):2256904. doi: 10.1080/21645515.2023.2256904. Epub 2023 Sep 29. Hum Vaccin Immunother. 2023. PMID: 37772505 Free PMC article. Review.
-
Informed by Cancer Stem Cells of Solid Tumors: Advances in Treatments Targeting Tumor-Promoting Factors and Pathways.Int J Mol Sci. 2024 Apr 7;25(7):4102. doi: 10.3390/ijms25074102. Int J Mol Sci. 2024. PMID: 38612911 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

