Intracellular interferon signalling pathways as potential regulators of covalently closed circular DNA in the treatment of chronic hepatitis B
- PMID: 33911462
- PMCID: PMC8047536
- DOI: 10.3748/wjg.v27.i14.1369
Intracellular interferon signalling pathways as potential regulators of covalently closed circular DNA in the treatment of chronic hepatitis B
Abstract
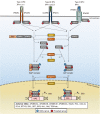
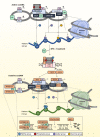
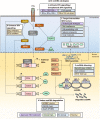
Infection with the hepatitis B virus (HBV) is still a major global health threat as 250 million people worldwide continue to be chronically infected with the virus. While patients may be treated with nucleoside/nucleotide analogues, this only suppresses HBV titre to sub-detection levels without eliminating the persistent HBV covalently closed circular DNA (cccDNA) genome. As a result, HBV infection cannot be cured, and the virus reactivates when conditions are favorable. Interferons (IFNs) are cytokines known to induce powerful antiviral mechanisms that clear viruses from infected cells. They have been shown to induce cccDNA clearance, but their use in the treatment of HBV infection is limited as HBV-targeting immune cells are exhausted and HBV has evolved multiple mechanisms to evade and suppress IFN signalling. Thus, to fully utilize IFN-mediated intracellular mechanisms to effectively eliminate HBV, instead of direct IFN administration, novel strategies to sustain IFN-mediated anti-cccDNA and antiviral mechanisms need to be developed. This review will consolidate what is known about how IFNs act to achieve its intracellular antiviral effects and highlight the critical interferon-stimulated gene targets and effector mechanisms with potent anti-cccDNA functions. These include cccDNA degradation by APOBECs and cccDNA silencing and transcription repression by epigenetic modifications. In addition, the mechanisms that HBV employs to disrupt IFN signalling will be discussed. Drugs that have been developed or are in the pipeline for components of the IFN signalling pathway and HBV targets that detract IFN signalling mechanisms will also be identified and discussed for utility in the treatment of HBV infections. Together, these will provide useful insights into design strategies that specifically target cccDNA for the eradication of HBV.
Keywords: APOBECs; Covalently closed circular DNA; Epigenetic modification; Hepatitis B virus therapeutics; Interferons.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Authors declare no conflicts-of-interest for this article.
Figures
Similar articles
-
Current Status and Challenges in Anti-Hepatitis B Virus Agents Based on Inactivation/Inhibition or Elimination of Hepatitis B Virus Covalently Closed Circular DNA.Viruses. 2023 Nov 25;15(12):2315. doi: 10.3390/v15122315. Viruses. 2023. PMID: 38140556 Free PMC article. Review.
-
Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication.J Hepatol. 2020 May;72(5):865-876. doi: 10.1016/j.jhep.2019.12.009. Epub 2019 Dec 18. J Hepatol. 2020. PMID: 31863794
-
Interferon Alpha Induces Multiple Cellular Proteins That Coordinately Suppress Hepadnaviral Covalently Closed Circular DNA Transcription.J Virol. 2020 Aug 17;94(17):e00442-20. doi: 10.1128/JVI.00442-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581092 Free PMC article.
-
Cellular DNA Topoisomerases Are Required for the Synthesis of Hepatitis B Virus Covalently Closed Circular DNA.J Virol. 2019 May 15;93(11):e02230-18. doi: 10.1128/JVI.02230-18. Print 2019 Jun 1. J Virol. 2019. PMID: 30867306 Free PMC article.
-
Antiviral strategies to eliminate hepatitis B virus covalently closed circular DNA (cccDNA).Curr Opin Pharmacol. 2016 Oct;30:144-150. doi: 10.1016/j.coph.2016.08.015. Curr Opin Pharmacol. 2016. PMID: 27639371 Review.
Cited by
-
Role of epigenetic modification in interferon treatment of hepatitis B virus infection.Front Immunol. 2022 Oct 17;13:1018053. doi: 10.3389/fimmu.2022.1018053. eCollection 2022. Front Immunol. 2022. PMID: 36325353 Free PMC article. Review.
-
Heterogeneity of immune control in chronic hepatitis B virus infection: Clinical implications on immunity with interferon-α treatment and retreatment.World J Gastroenterol. 2022 Oct 28;28(40):5784-5800. doi: 10.3748/wjg.v28.i40.5784. World J Gastroenterol. 2022. PMID: 36353205 Free PMC article. Review.
-
The efficacy of pegylated interferon alpha-2a and entecavir in HBeAg-positive children and adolescents with chronic hepatitis B.BMC Pediatr. 2022 Jul 20;22(1):426. doi: 10.1186/s12887-022-03482-0. BMC Pediatr. 2022. PMID: 35854256 Free PMC article.
References
-
- World Health Organization. Hepatitis B, 2020 [cited Feb 23, 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b .
-
- Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. - PubMed
-
- Alter H, Block T, Brown N, Brownstein A, Brosgart C, Chang KM, Chen PJ, Chisari FV, Cohen C, El-Serag H, Feld J, Gish R, Glenn J, Greten T, Guo H, Guo JT, Hoshida Y, Hu J, Kowdley KV, Li W, Liang J, Locarnini S, Lok AS, Mason W, McMahon B, Mehta A, Perrillo R, Revill P, Rice CM, Rinaudo J, Schinazi R, Seeger C, Shetty K, Tavis J, Zoulim F. A research agenda for curing chronic hepatitis B virus infection. Hepatology. 2018;67:1127–1131. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

