Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy
- PMID: 33910093
- PMCID: PMC8285002
- DOI: 10.1016/j.freeradbiomed.2021.03.046
Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy
Abstract
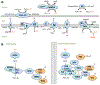
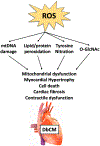
Even in the absence of coronary artery disease and hypertension, diabetes mellitus (DM) may increase the risk for heart failure development. This risk evolves from functional and structural alterations induced by diabetes in the heart, a cardiac entity termed diabetic cardiomyopathy (DbCM). Oxidative stress, defined as the imbalance of reactive oxygen species (ROS) has been increasingly proposed to contribute to the development of DbCM. There are several sources of ROS production including the mitochondria, NAD(P)H oxidase, xanthine oxidase, and uncoupled nitric oxide synthase. Overproduction of ROS in DbCM is thought to be counterbalanced by elevated antioxidant defense enzymes such as catalase and superoxide dismutase. Excess ROS in the cardiomyocyte results in further ROS production, mitochondrial DNA damage, lipid peroxidation, post-translational modifications of proteins and ultimately cell death and cardiac dysfunction. Furthermore, ROS modulates transcription factors responsible for expression of antioxidant enzymes. Lastly, evidence exists that several pharmacological agents may convey cardiovascular benefit by antioxidant mechanisms. As such, increasing our understanding of the pathways that lead to increased ROS production and impaired antioxidant defense may enable the development of therapeutic strategies against the progression of DbCM. Herein, we review the current knowledge about causes and consequences of ROS in DbCM, as well as the therapeutic potential and strategies of targeting oxidative stress in the diabetic heart.
Keywords: Diabetes; Diabetic cardiomyopathy; Diabetic heart; Mitochondria; Oxidative stress; Reactive oxygen species.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential.Pharmacol Ther. 2017 Apr;172:50-62. doi: 10.1016/j.pharmthera.2016.11.013. Epub 2016 Dec 1. Pharmacol Ther. 2017. PMID: 27916650 Review.
-
Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy.Free Radic Biol Med. 2016 Jan;90:12-23. doi: 10.1016/j.freeradbiomed.2015.11.013. Epub 2015 Nov 11. Free Radic Biol Med. 2016. PMID: 26577173 Free PMC article.
-
KLF5 Is Induced by FOXO1 and Causes Oxidative Stress and Diabetic Cardiomyopathy.Circ Res. 2021 Feb 5;128(3):335-357. doi: 10.1161/CIRCRESAHA.120.316738. Epub 2020 Dec 2. Circ Res. 2021. PMID: 33539225 Free PMC article.
-
Role of antioxidants in redox regulation of diabetic cardiovascular complications.Curr Pharm Biotechnol. 2010 Dec;11(8):819-36. doi: 10.2174/138920110793262123. Curr Pharm Biotechnol. 2010. PMID: 20874678 Review.
-
Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110α) signaling.Free Radic Biol Med. 2015 Oct;87:137-47. doi: 10.1016/j.freeradbiomed.2015.04.028. Epub 2015 Apr 30. Free Radic Biol Med. 2015. PMID: 25937176
Cited by
-
[Resveratrol alleviates hyperglycemia-induced cardiomyocyte hypertrophy by maintaining mitochondrial homeostasis via enhancing SIRT1 expression].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Jan 20;44(1):45-51. doi: 10.12122/j.issn.1673-4254.2024.01.06. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 38293975 Free PMC article. Chinese.
-
Ferroptosis in diabetic cardiomyopathy: Advances in cardiac fibroblast-cardiomyocyte interactions.Heliyon. 2024 Jul 28;10(15):e35219. doi: 10.1016/j.heliyon.2024.e35219. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39165946 Free PMC article. Review.
-
1,25(OH)2D3 ameliorates doxorubicin‑induced cardiomyopathy by inhibiting the NLRP3 inflammasome and oxidative stress.Exp Ther Med. 2023 Jul 11;26(3):413. doi: 10.3892/etm.2023.12112. eCollection 2023 Sep. Exp Ther Med. 2023. PMID: 37559932 Free PMC article.
-
Zataria multiflora and its constituent, carvacrol, counteract sepsis-induced aortic and cardiac toxicity in rat: Involvement of nitric oxide and oxidative stress.Animal Model Exp Med. 2023 Jun;6(3):221-229. doi: 10.1002/ame2.12323. Epub 2023 Jun 5. Animal Model Exp Med. 2023. PMID: 37272426 Free PMC article.
-
Novel insights into the role of mitochondria in diabetic cardiomyopathy: molecular mechanisms and potential treatments.Cell Stress Chaperones. 2023 Nov;28(6):641-655. doi: 10.1007/s12192-023-01361-w. Epub 2023 Jul 5. Cell Stress Chaperones. 2023. PMID: 37405612 Free PMC article. Review.
References
-
- Kristensen SL, et al., Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: a report from the I-preserve trial (irbesartan in heart failure with preserved ejection, Circulation (2017), 10.1161/CIRCULATIONAHA.116.024593. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

