Disarranged neuroplastin environment upon aging and chronic stress recovery in female Sprague Dawley rats
- PMID: 33909305
- PMCID: PMC9290558
- DOI: 10.1111/ejn.15256
Disarranged neuroplastin environment upon aging and chronic stress recovery in female Sprague Dawley rats
Abstract
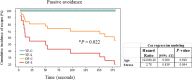
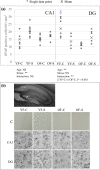
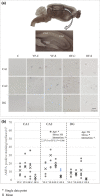
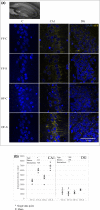
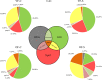
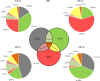
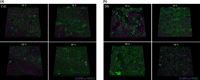
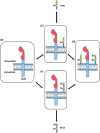
Chronic stress produces long-term metabolic changes throughout the superfamily of nuclear receptors, potentially causing various pathologies. Sex hormones modulate the stress response and generate a sex-specific age-dependent metabolic imprint, especially distinct in the reproductive senescence of females. We monitored chronic stress recovery in two age groups of female Sprague Dawley rats to determine whether stress and/or aging structurally changed the glycolipid microenvironment, a milieu playing an important role in cognitive functions. Old females experienced memory impairment even at basal conditions, which was additionally amplified by stress. On the other hand, the memory of young females was not disrupted. Stress recovery was followed by a microglial decrease and an increase in astrocyte count in the hippocampal immune system. Since dysfunction of the brain immune system could contribute to disturbed synaptogenesis, we analyzed neuroplastin expression and the lipid environment. Neuroplastin microenvironments were explored by analyzing immunofluorescent stainings using a newly developed Python script method. Stress reorganized glycolipid microenvironment in the Cornu Ammonis 1 (CA1) and dentate gyrus (DG) hippocampal regions of old females but in a very different fashion, thus affecting neuroplasticity. The postulation of four possible neuroplastin environments pointed to the GD1a ganglioside enrichment during reproductive senescence of stressed females, as well as its high dispersion in both regions and to GD1a and GM1 loss in the CA1 region. A specific lipid environment might influence neuroplastin functionality and underlie synaptic dysfunction triggered by a combination of aging and chronic stress.
Keywords: GD1a; GM1; hippocampus; neurodegeneration; neuroinflammation; reproductive senescence.
© 2021 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


Similar articles
-
Neuroplastin in human cognition: review of literature and future perspectives.Transl Psychiatry. 2021 Jul 16;11(1):394. doi: 10.1038/s41398-021-01509-1. Transl Psychiatry. 2021. PMID: 34282131 Free PMC article. Review.
-
Impact of sex and hypoxia on brain region-specific expression of membrane androgen receptor AR45 in rats.Front Endocrinol (Lausanne). 2024 Jul 18;15:1420144. doi: 10.3389/fendo.2024.1420144. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39092288 Free PMC article.
-
The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses.Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4327-32. doi: 10.1073/pnas.080389297. Proc Natl Acad Sci U S A. 2000. PMID: 10759566 Free PMC article.
-
Hippocampal Subregion Transcriptomic Profiles Reflect Strategy Selection during Cognitive Aging.J Neurosci. 2020 Jun 17;40(25):4888-4899. doi: 10.1523/JNEUROSCI.2944-19.2020. Epub 2020 May 6. J Neurosci. 2020. PMID: 32376783 Free PMC article.
-
Hippocampal expression of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer's disease.J Cell Mol Med. 2019 Feb;23(2):1602-1607. doi: 10.1111/jcmm.13998. Epub 2018 Nov 28. J Cell Mol Med. 2019. PMID: 30488668 Free PMC article.
Cited by
-
Plasma Membrane Calcium ATPase-Neuroplastin Complexes Are Selectively Stabilized in GM1-Containing Lipid Rafts.Int J Mol Sci. 2021 Dec 18;22(24):13590. doi: 10.3390/ijms222413590. Int J Mol Sci. 2021. PMID: 34948386 Free PMC article.
-
Neuroplastin in human cognition: review of literature and future perspectives.Transl Psychiatry. 2021 Jul 16;11(1):394. doi: 10.1038/s41398-021-01509-1. Transl Psychiatry. 2021. PMID: 34282131 Free PMC article. Review.
-
Neuroplastin in Neuropsychiatric Diseases.Genes (Basel). 2021 Sep 26;12(10):1507. doi: 10.3390/genes12101507. Genes (Basel). 2021. PMID: 34680901 Free PMC article. Review.
-
Lipid Rafts: The Maestros of Normal Brain Development.Biomolecules. 2024 Mar 18;14(3):362. doi: 10.3390/biom14030362. Biomolecules. 2024. PMID: 38540780 Free PMC article. Review.
References
-
- An, G. H. , Chen, X. W. , Li, C. , Zhang, L. I. , Wei, M. F. , Chen, J. J. , Ma, Q. , Yang, D. F. , & Wang, J. (2020). Pathophysiological changes in female rats with estrous cycle disorder induced by long‐term heat stress. BioMed Research International, 2020, 4701563. 10.1155/2020/4701563 - DOI - PMC - PubMed
-
- Balog, M. , Miljanović, M. , Blažetić, S. , Labak, I. , Ivić, V. , Viljetić, B. , & Heffer, M. (2015). Sex‐specific chronic stress response at the level of adrenal gland modified sexual hormone and leptin receptors. Croatian Medical Journal, 56(2), 104–113. 10.3325/cmj.2015.56.104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

