Inflammasome as an Effective Platform for Fibrosis Therapy
- PMID: 33907438
- PMCID: PMC8069677
- DOI: 10.2147/JIR.S304180
Inflammasome as an Effective Platform for Fibrosis Therapy
Abstract
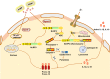
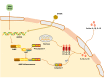
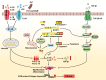
Fibrosis is the final stage of the development of chronic inflammation. It is characterized by excessive deposition of the extracellular matrix, leading to tissue structure damage and organ dysfunction, which is a serious threat to human health and life. However, the molecular mechanism of fibrosis is still unclear. Inflammasome is a molecular complex of proteins that has been becoming a key innate sensor for host immunity and is involved in pyroptosis, pathogen infection, metabolic syndrome, cellular stress, and tumor metastasis. Inflammasome signaling and downstream cytokine responses mediated by the inflammasome have been found to play an important role in fibrosis. The inflammasome regulates the secretion of IL-1β and IL-18, which are both critical for the process of fibrosis. Recently, researches on the function of inflammasome have attracted extensive attention, and data derived from these researches have increased our understanding of the effects and regulation of inflammasome during fibrosis. In this review, we emphasize the growing evidence for both indirect and direct effects of inflammasomes in triggering fibrosis as well as potential novel targets for antifibrotic therapies.
Keywords: AIM2; NLRP3; caspase-1; fibrosis; inflammasome.
© 2021 Chen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury.Brain Res. 2018 Oct 15;1697:10-20. doi: 10.1016/j.brainres.2018.06.008. Epub 2018 Jun 8. Brain Res. 2018. PMID: 29886252
-
The role of NLRP3 and AIM2 in inflammasome activation during Brucella abortus infection.Semin Immunopathol. 2017 Feb;39(2):215-223. doi: 10.1007/s00281-016-0581-1. Epub 2016 Jul 12. Semin Immunopathol. 2017. PMID: 27405866 Free PMC article. Review.
-
The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis.J Biol Chem. 2020 Jan 24;295(4):1120-1141. doi: 10.1074/jbc.RA119.011751. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852739 Free PMC article.
-
Role of Inflammasome in Chronic Kidney Disease.Adv Exp Med Biol. 2019;1165:407-421. doi: 10.1007/978-981-13-8871-2_19. Adv Exp Med Biol. 2019. PMID: 31399976 Review.
Cited by
-
D-Carvone Attenuates CCl4-Induced Liver Fibrosis in Rats by Inhibiting Oxidative Stress and TGF-ß 1/SMAD3 Signaling Pathway.Biology (Basel). 2022 May 12;11(5):739. doi: 10.3390/biology11050739. Biology (Basel). 2022. PMID: 35625467 Free PMC article.
-
Regulatory Cues in Pulmonary Fibrosis-With Emphasis on the AIM2 Inflammasome.Int J Mol Sci. 2023 Jun 29;24(13):10876. doi: 10.3390/ijms241310876. Int J Mol Sci. 2023. PMID: 37446052 Free PMC article. Review.
-
Polarization Behavior of Bone Macrophage as Well as Associated Osteoimmunity in Glucocorticoid-Induced Osteonecrosis of the Femoral Head.J Inflamm Res. 2023 Feb 28;16:879-894. doi: 10.2147/JIR.S401968. eCollection 2023. J Inflamm Res. 2023. PMID: 36891172 Free PMC article. Review.
-
Advances in the Pathogenesis of Steroid-Associated Osteonecrosis of the Femoral Head.Biomolecules. 2024 Jun 6;14(6):667. doi: 10.3390/biom14060667. Biomolecules. 2024. PMID: 38927070 Free PMC article. Review.
-
Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis.J Zhejiang Univ Sci B. 2023 Jul 1;24(8):723-733. doi: 10.1631/jzus.B2200385. J Zhejiang Univ Sci B. 2023. PMID: 37551558 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

