Long-Acting Bronchodilator Use in Chronic Obstructive Pulmonary Disease in Primary Care in New Zealand: A Retrospective Study of Treatment Patterns and Evolution Using the HealthStat Database
- PMID: 33907394
- PMCID: PMC8068498
- DOI: 10.2147/COPD.S290887
Long-Acting Bronchodilator Use in Chronic Obstructive Pulmonary Disease in Primary Care in New Zealand: A Retrospective Study of Treatment Patterns and Evolution Using the HealthStat Database
Abstract
Purpose: Long-acting bronchodilator (LABD) use is the mainstay of pharmacologic treatment for chronic obstructive pulmonary disease (COPD). Few studies describe evolving patterns of LABD use in the setting of changing inhaler availability and updated clinical guidelines.
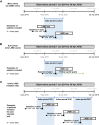
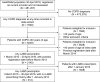
Methods: A retrospective cohort study in New Zealand using the HealthStat general practice database (01/2014 to 04/2018). Eligible patients (aged ≥40 years) had COPD and ≥1 LABD prescription (long-acting muscarinic antagonist [LAMA] and/or long-acting β2-agonist [LABA]) during the index period (05/2015 to 04/2016). Demographics and clinical characteristics of all LABD users (overall/by treatment) were described at baseline. Patients starting LABD treatment during the index period, termed "new" users, were also described, as was their treatment evolution over 24 months of follow-up. Yearly LABD initiation rates were assessed from 2015 to 2017, covering changes to Pharmaceutical Management Agency criteria and clinical guidelines.
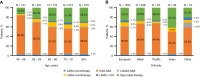
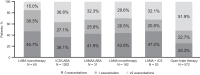
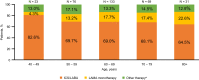
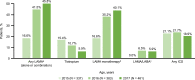
Results: Across 2140 eligible patients, the most common index treatments were inhaled corticosteroid (ICS)/LABA (59.0%) and open triple therapy (LAMA+LABA+ICS; 26.7%). ICS/LABA therapy was highest in younger patients, with open triple therapy highest in older patients. Prior yearly exacerbation rates were lowest in those receiving monotherapy (LABA: 0.9/year; LAMA: 1.1/year) versus dual therapy (all 1.4/year) and open triple therapy (2.2/year). Of 312 new LABD users, ICS/LABA was the most common index treatment (69.6%), followed by LAMA monotherapy (16.0%). Continuous use with index treatment was 31.1% at 12 months and 13.5% at 24 months; mean time to treatment change was 175.5 and 244.1 days, respectively. Among patients modifying treatment at 24 months, 23.0% augmented, 7.0% switched, 45.6% re-started, and 24.4% discontinued/stepped down. Among patients initiating LABD each year from 2015 to 2017, LAMA prescription increased (17% to 46%) while ICS prescription remained stable (approximately 20%).
Conclusion: Predominant use of ICS/LABA (05/2015 to 04/2016) reflects available LABDs and previous restrictions on LAMA use in New Zealand.
Keywords: New Zealand; bronchodilator therapy; chronic obstructive pulmonary disease; inhaled corticosteroid; long-acting muscarinic antagonist; long-acting β2-agonist.
© 2021 Milea et al.
Conflict of interest statement
DM, AANR, SS, and BM are employees of, and shareholders in, GlaxoSmithKline plc. S-HY and JB are former employees of GlaxoSmithKline plc. YN is a former employee of Adelphi Real World, who received funding from GlaxoSmithKline plc. to conduct this study. RPY and RJS have received honorarium from GlaxoSmithKline plc. for educational talks and participation in advisory groups. BG is an employee of CBG Health Research Ltd, who received funding from GlaxoSmithKline plc. to conduct this study. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
COPD treatment pathways in France: a retrospective analysis of electronic medical record data from general practitioners.Int J Chron Obstruct Pulmon Dis. 2018 Dec 18;14:51-63. doi: 10.2147/COPD.S181224. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30587961 Free PMC article.
-
A Real-World Analysis of Treatment Patterns and Clinical Characteristics Among Patients with COPD Who Initiated Multiple-Inhaler Triple Therapy in New Zealand.Int J Chron Obstruct Pulmon Dis. 2021 Jun 18;16:1835-1850. doi: 10.2147/COPD.S295183. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34177262 Free PMC article.
-
Comparative Effectiveness of Long-Acting Beta2 -Agonist Combined with a Long-Acting Muscarinic Antagonist or Inhaled Corticosteroid in Chronic Obstructive Pulmonary Disease.Pharmacotherapy. 2017 Apr;37(4):447-455. doi: 10.1002/phar.1913. Pharmacotherapy. 2017. PMID: 28226405
-
Long-acting muscarinic antagonist and long-acting β2-agonist combination for the treatment of maintenance therapy-naïve patients with chronic obstructive pulmonary disease: a narrative review.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241279115. doi: 10.1177/17534666241279115. Ther Adv Respir Dis. 2024. PMID: 39352722 Free PMC article. Review.
-
Role of Long-Acting Muscarinic Antagonist/Long-Acting β2-Agonist Therapy in Chronic Obstructive Pulmonary Disease.Ann Pharmacother. 2017 Aug;51(8):696-705. doi: 10.1177/1060028017705149. Epub 2017 Apr 14. Ann Pharmacother. 2017. PMID: 28410560 Review.
Cited by
-
The Impact of the GOLD 2023 on Clinical Treatment in Northeast China.Int J Chron Obstruct Pulmon Dis. 2023 Nov 30;18:2819-2823. doi: 10.2147/COPD.S418971. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 38053920 Free PMC article. No abstract available.
-
Methods to assess COPD medications adherence in healthcare databases: a systematic review.Eur Respir Rev. 2023 Sep 27;32(169):230103. doi: 10.1183/16000617.0103-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37758274 Free PMC article. Review.
-
Transforming Primary Care Data Into the Observational Medical Outcomes Partnership Common Data Model: Development and Usability Study.JMIR Med Inform. 2024 Aug 13;12:e49542. doi: 10.2196/49542. JMIR Med Inform. 2024. PMID: 39140273 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report); 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25.... Accessed February4, 2021. - PubMed
-
- Cazzola M, Page C. Long-acting bronchodilators in COPD: where are we now and where are we going? Breath. 2014;10(2):111–120. doi:10.1183/20734735.014813 - DOI
-
- Barnard L, Zhang J. The impact of respiratory disease in New Zealand: 2018 update. Asthma + Respiratory Foundation NZ; 2019.
-
- Yang IA, Brown JL, George J, et al. The COPD-X plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease; 2020. Available from: https://copdx.org.au/copd-x-plan/Last. Accessed February4, 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

