Effectiveness and Safety of the Clareon Monofocal Intraocular Lens: Outcomes from a 12-Month Single-Arm Clinical Study in a Large Sample
- PMID: 33907378
- PMCID: PMC8068507
- DOI: 10.2147/OPTH.S295008
Effectiveness and Safety of the Clareon Monofocal Intraocular Lens: Outcomes from a 12-Month Single-Arm Clinical Study in a Large Sample
Abstract
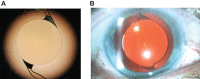
Purpose: This study assessed effectiveness and safety of the novel Clareon intraocular lens (IOL; model SY60CL; Alcon Vision LLC).
Patients and methods: This was a prospective, single-arm, unmasked clinical trial at 16 investigative clinical sites in the United States. Included were adults ≥22 years who required cataract extraction by phacoemulsification. Following phacoemulsification, 350 subjects received SY60CL IOL unilaterally; 342 completed the study. Monocular best corrected distance visual acuity (CDVA) and uncorrected distance visual acuity (UDVA) were evaluated. The primary effectiveness endpoint was the percentage of subjects with CDVA ≤0.3 logMAR at month 12. Safety was assessed by monitoring adverse events (AEs). Visual acuity and safety outcomes were compared with historical safety and performance endpoint (SPE) rates.
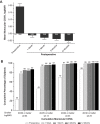
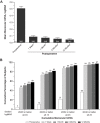
Results: At 12 months post-implantation, 99.7% of subjects receiving the SY60CL IOL achieved monocular CDVA ≤0.3 logMAR (primary effectiveness endpoint; 1-sided 95% upper confidence limit >SPE rate); 99.7% and 86.8% of subjects achieved monocular CDVA of ≤0.34 (20/40 Snellen or better) and ≤0.04 logMAR (20/20 Snellen or better), respectively. At 12 months, >95% of subjects achieved mean monocular UDVA ≤0.3 logMAR; 97.1% and 57.6% of subjects achieved monocular CDVA of ≤0.34 and ≤0.04 logMAR, respectively. Mean monocular CDVA and UDVA were -0.05 and 0.04 logMAR, respectively. AEs were within SPE limits. The most common nonserious ocular AE was posterior capsule opacification (5.4%). Serious AEs were <1%, and no serious ocular AEs were assessed as related to the device. There were no observations for IOL glistenings at 12 months.
Conclusion: Results of this study supported effectiveness and safety of the SY60CL IOL. Visual acuity outcomes with the SY60CL IOL exceeded the SPE rates for monocular CDVA and AEs were within the limit of historic SPE rates. (Model number SY60WF is the Clareon lens approved by the FDA.).
Keywords: cystoid macular edema; dysphotopsia; glistenings; posterior capsule opacification; visual acuity.
© 2021 Lehmann et al.
Conflict of interest statement
RL, AM, and DML are Alcon consultants. AF is a researcher and a speaker for Alcon. Dr David M Lubeck reports he is a consultant for NovaEye, consultant/investigator for Glaukos, outside the submitted work. Dr Andrew Maxwell reports personal fees from Alcon, outside the submitted work. Dr Robert Lehmann reports grants from Alcon, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Twelve-Months Follow-Up Postmarket Study of a Hydrophobic Intraocular Lens Using a Preloaded Automated Injector in an Indian Population.Clin Ophthalmol. 2022 Dec 16;16:4215-4225. doi: 10.2147/OPTH.S379054. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36561374 Free PMC article. Clinical Trial.
-
Three-year multinational clinical study on an aspheric hydrophobic acrylic intraocular lens.J Cataract Refract Surg. 2023 Jul 1;49(7):672-678. doi: 10.1097/j.jcrs.0000000000001173. Epub 2023 Feb 22. J Cataract Refract Surg. 2023. PMID: 36848238 Free PMC article.
-
Visual and Patient-Reported Outcomes of a Diffractive Trifocal Intraocular Lens Compared with Those of a Monofocal Intraocular Lens.Ophthalmology. 2021 Feb;128(2):197-207. doi: 10.1016/j.ophtha.2020.07.015. Epub 2020 Sep 28. Ophthalmology. 2021. PMID: 33004211 Clinical Trial.
-
Long-term real-life outcomes of the Clareon® hydrophobic intraocular lens: the Clarte study in 191 eyes : 3-years real-life outcomes of the Clareon® intraocular lens.BMC Ophthalmol. 2024 Mar 26;24(1):133. doi: 10.1186/s12886-024-03393-x. BMC Ophthalmol. 2024. PMID: 38532367 Free PMC article.
-
[Clinical experience with the Clareon® IOL and the AutonoMe® implantation system].Ophthalmologe. 2020 Nov;117(11):1100-1104. doi: 10.1007/s00347-020-01075-9. Ophthalmologe. 2020. PMID: 32112221 Review. German.
Cited by
-
Rotational Stability of the Clareon Monofocal Aspheric Hydrophobic Acrylic Intraocular Lens 6 Months After Implantation.Clin Ophthalmol. 2022 Feb 15;16:401-409. doi: 10.2147/OPTH.S348551. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35210745 Free PMC article.
-
Twelve-Months Follow-Up Postmarket Study of a Hydrophobic Intraocular Lens Using a Preloaded Automated Injector in an Indian Population.Clin Ophthalmol. 2022 Dec 16;16:4215-4225. doi: 10.2147/OPTH.S379054. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36561374 Free PMC article. Clinical Trial.
-
Comparison Between L-312 Hydrophobic-Hydrophilic Acrylate and US-860 UV Hydrophilic Acrylate IOL Opacification Characteristic.Front Med (Lausanne). 2022 Apr 8;9:873684. doi: 10.3389/fmed.2022.873684. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35463016 Free PMC article.
-
Evaluation of Intermediate Visual Outcomes in Eyes Implanted with Bilateral Advanced Monofocal Intraocular Lens Targeting for Mini-Monovision and Its Association with Age and Corneal Asphericity.Clin Ophthalmol. 2024 Oct 16;18:2929-2937. doi: 10.2147/OPTH.S484030. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39429441 Free PMC article.
-
Clinical Evaluation of a Hydrophobic Intraocular Lens Using a Preloaded Automated Injector in a Korean Population.Clin Ophthalmol. 2023 Nov 3;17:3353-3363. doi: 10.2147/OPTH.S421864. eCollection 2023. Clin Ophthalmol. 2023. PMID: 37941777 Free PMC article.
References
-
- Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487–494. - PubMed
-
- Lundstrom M, Barry P, Henry Y, Rosen P, Stenevi U. Evidence-based guidelines for cataract surgery: guidelines based on data in the European Registry of Quality Outcomes for Cataract and Refractive Surgery database. J Cataract Refract Surg. 2012;38(6):1086–1093. doi:10.1016/j.jcrs.2012.03.006 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

