Homeostasis and Durability of T-Cell Memory-The Resting and the Restless T-Cell Memory
- PMID: 33903153
- PMCID: PMC8247562
- DOI: 10.1101/cshperspect.a038083
Homeostasis and Durability of T-Cell Memory-The Resting and the Restless T-Cell Memory
Abstract
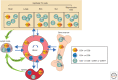
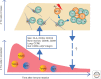
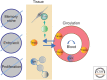
The molecular basis of the persistence of experienced T lymphocytes, also known as "memory T lymphocytes," is still enigmatic. We are beginning to understand their considerable heterogeneity and topographic compartmentalization into memory T cells circulating through the body and those residing in a particular tissue. In some tissues, like murine spleen, subpopulations of memory T cells proliferating in the absence of antigen (homeostatic proliferation) have been described. Other populations are maintained resting in terms of transcription, mobility, and proliferation in dedicated survival niches organized by stromal cells. The survival of these memory T cells is conditional on being in such a niche, where they can persist for a lifetime. Circulating memory T lymphocytes of distinct immune responses slowly decline in numbers over time. The rules governing their entry into and exit from blood, as well as their lifestyle outside of the blood and their relation to resident memory T cells are poorly understood. Homeostasis of circulating, proliferating, and resting memory T cells is obviously controlled by different rheostats: tissue-exit and tissue-entry signals for circulating and proliferation-inducing signals for proliferating memory T cells. For tissue-resident, resting memory T cells, it is the availability of their survival niche. Apparently, this mechanism (i.e., the link between memory T cell and stromal cell) is so robust that it provides efficient T-cell memory over a lifetime in tissues such as the bone marrow.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

Similar articles
-
Immunological memories of the bone marrow.Immunol Rev. 2018 May;283(1):86-98. doi: 10.1111/imr.12656. Immunol Rev. 2018. PMID: 29664564 Free PMC article. Review.
-
Maintenance of quiescent immune memory in the bone marrow.Eur J Immunol. 2021 Jul;51(7):1592-1601. doi: 10.1002/eji.202049012. Epub 2021 Jun 1. Eur J Immunol. 2021. PMID: 34010475 Review.
-
Organization and maintenance of immunological memory by stroma niches.Eur J Immunol. 2009 Aug;39(8):2095-9. doi: 10.1002/eji.200939500. Eur J Immunol. 2009. PMID: 19637201 Review.
-
Maintenance of CD8+ memory T lymphocytes in the spleen but not in the bone marrow is dependent on proliferation.Eur J Immunol. 2017 Nov;47(11):1900-1905. doi: 10.1002/eji.201747063. Epub 2017 Oct 11. Eur J Immunol. 2017. PMID: 28815584 Free PMC article.
-
Memory CD8(+) T cells colocalize with IL-7(+) stromal cells in bone marrow and rest in terms of proliferation and transcription.Eur J Immunol. 2015 Apr;45(4):975-87. doi: 10.1002/eji.201445295. Epub 2015 Feb 27. Eur J Immunol. 2015. PMID: 25639669 Free PMC article.
Cited by
-
Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition).Eur J Immunol. 2021 Dec;51(12):2708-3145. doi: 10.1002/eji.202170126. Epub 2021 Dec 7. Eur J Immunol. 2021. PMID: 34910301 Free PMC article. Review.
-
Effect of cellular aging on memory T-cell homeostasis.Front Immunol. 2022 Aug 8;13:947242. doi: 10.3389/fimmu.2022.947242. eCollection 2022. Front Immunol. 2022. PMID: 36059495 Free PMC article.
-
Caveolin-1 modulates cisplatin sensitivity in oral squamous cell carcinoma through ferroptosis.Clin Transl Oncol. 2024 Sep 26. doi: 10.1007/s12094-024-03724-w. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39322925
-
Counteracting Immunosuppression in the Tumor Microenvironment by Oncolytic Newcastle Disease Virus and Cellular Immunotherapy.Int J Mol Sci. 2022 Oct 27;23(21):13050. doi: 10.3390/ijms232113050. Int J Mol Sci. 2022. PMID: 36361831 Free PMC article. Review.
-
A ubiquitous bone marrow reservoir of preexisting SARS-CoV-2-reactive memory CD4+ T lymphocytes in unexposed individuals.Front Immunol. 2022 Oct 4;13:1004656. doi: 10.3389/fimmu.2022.1004656. eCollection 2022. Front Immunol. 2022. PMID: 36268016 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
