Synovial Immunohistological Biomarkers of the Classification of Undifferentiated Arthritis Evolving to Rheumatoid or Psoriatic Arthritis
- PMID: 33898490
- PMCID: PMC8062857
- DOI: 10.3389/fmed.2021.656667
Synovial Immunohistological Biomarkers of the Classification of Undifferentiated Arthritis Evolving to Rheumatoid or Psoriatic Arthritis
Abstract
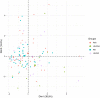
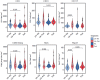
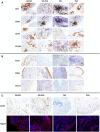
Background: Undifferentiated arthritis (UA) is defined as an inflammatory arthritis that does not fulfill criteria for a definite diagnosis. Delay in reaching a specific diagnostic and therapy may lead to impaired functional outcomes. Our aim was to identify synovial biomarkers associated with definitive diagnostic classification in patients with UA. Methods: DMARD-naïve UA patients with available initial synovial tissue (ST) and a final diagnosis of rheumatoid arthritis (RA) or psoriatic arthritis (PsA) during follow-up were included and compared with patients with well-defined disease (RA or PsA). Clinical, arthroscopic, and pathological data were compared between groups. Pathology included quantitative immunohistochemical (IHC) analysis of cell types and human interferon-regulated MxA. Principal component analysis (PCA) was performed to extract disease patterns. Results: One hundred and five patients were included: 31 patients with DMARD-naïve UA (19 evolving to RA and 12 to PsA during a median follow up of 7 years), 39 with established RA, and 35 with established PsA. ST from the UA group showed higher macrophage density compared with the established RA and PsA groups. Patients with UA evolving to RA (UA-RA) showed higher MxA expression and CD3+ T-cell density compared with established RA. UA patients evolving to PsA (UA-PsA) showed increased vascularity and lining synovial fibroblast density compared with established PsA. Synovitis of UA-PsA patients showed more mast cells and lining fibroblasts compared with UA-RA. No between-group differences in local or systemic inflammation markers were found. Conclusions: Our results show differences in the cellular composition of UA synovium compared with RA and PsA, with higher density of the cellular infiltrate in the UA groups. Initial expression of the interferon inducible gene MxA could be a biomarker of progression to RA, while higher mast cell and fibroblastic density may be associated with PsA progression.
Keywords: biomarkers; psoriatic arthritis; rheumatoid arthritis; synovitis; undifferentiated arthritis.
Copyright © 2021 Cuervo, Celis, Julià, Usategui, Faré, Ramírez, Azuaga, Lorenzo, Sanmartí, Pablos and Cañete.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Synovial tissue features associated with poor prognosis in inflammatory arthritis.Arthritis Res Ther. 2024 Jan 10;26(1):18. doi: 10.1186/s13075-023-03255-9. Arthritis Res Ther. 2024. PMID: 38200561 Free PMC article.
-
GM-CSF Expression and Macrophage Polarization in Joints of Undifferentiated Arthritis Patients Evolving to Rheumatoid Arthritis or Psoriatic Arthritis.Front Immunol. 2021 Feb 17;11:613975. doi: 10.3389/fimmu.2020.613975. eCollection 2020. Front Immunol. 2021. PMID: 33679701 Free PMC article.
-
Differential synovial tissue biomarkers among psoriatic arthritis and rheumatoid factor/anti-citrulline antibody-negative rheumatoid arthritis.Arthritis Res Ther. 2019 May 9;21(1):116. doi: 10.1186/s13075-019-1898-7. Arthritis Res Ther. 2019. PMID: 31072400 Free PMC article.
-
Psoriatic Synovitis: Singularity and Potential Clinical Implications.Front Med (Lausanne). 2019 Feb 11;6:14. doi: 10.3389/fmed.2019.00014. eCollection 2019. Front Med (Lausanne). 2019. PMID: 30805340 Free PMC article. Review.
-
Synovial Fluid and Serum Concentrations of Inflammatory Markers in Rheumatoid Arthritis, Psoriatic Arthritis and Osteoarthitis: A Systematic Review.Curr Rheumatol Rev. 2017;13(3):170-179. doi: 10.2174/1573397113666170427125918. Curr Rheumatol Rev. 2017. PMID: 28460627 Review.
Cited by
-
Biomarkers in psoriatic arthritis: A meta-analysis and systematic review.Front Immunol. 2022 Nov 30;13:1054539. doi: 10.3389/fimmu.2022.1054539. eCollection 2022. Front Immunol. 2022. PMID: 36532039 Free PMC article.
-
Psoriatic Arthritis: Pathogenesis and Targeted Therapies.Int J Mol Sci. 2023 Mar 3;24(5):4901. doi: 10.3390/ijms24054901. Int J Mol Sci. 2023. PMID: 36902329 Free PMC article. Review.
-
Characterisation of prodromal and very early psoriatic arthritis: a systematic literature review informing a EULAR taskforce.RMD Open. 2023 Jun;9(2):e003143. doi: 10.1136/rmdopen-2023-003143. RMD Open. 2023. PMID: 37349122 Free PMC article.
-
The Crucial Questions on Synovial Biopsy: When, Why, Who, What, Where, and How?Front Med (Lausanne). 2021 Aug 6;8:705382. doi: 10.3389/fmed.2021.705382. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34422862 Free PMC article. Review.
-
Synovial tissue features associated with poor prognosis in inflammatory arthritis.Arthritis Res Ther. 2024 Jan 10;26(1):18. doi: 10.1186/s13075-023-03255-9. Arthritis Res Ther. 2024. PMID: 38200561 Free PMC article.
References
-
- Buch MH, Hensor EM, Rakieh C, Freeston JE, Middleton E, Horton S, et al. . Abatacept reduces disease activity and ultrasound power Doppler in ACPA-negative undifferentiated arthritis: a proof-of-concept clinical and imaging study. Rheumatology (Oxford). (2017) 56:58–67. 10.1093/rheumatology/kew357 - DOI - PubMed
-
- Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. . The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. (2011) 70:25–31. 10.1136/ard.2010.133645 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

