The Interplay of HIV-1 and Macrophages in Viral Persistence
- PMID: 33897659
- PMCID: PMC8058371
- DOI: 10.3389/fmicb.2021.646447
The Interplay of HIV-1 and Macrophages in Viral Persistence
Abstract
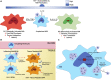
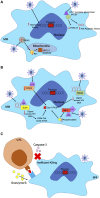
HIV-1 has evolved mechanisms to evade host cell immune responses and persist for lifelong infection. Latent cellular reservoirs are responsible for this persistence of HIV-1 despite the powerful effects of highly active antiretroviral therapies (HAART) to control circulating viral load. While cellular reservoirs have been extensively studied, much of these studies have focused on peripheral blood and resting memory CD4+ T cells containing latent HIV-1 provirus; however, efforts to eradicate cellular reservoirs have been stunted by reservoirs found in tissues compartments that are not easily accessible. These tissues contain resting memory CD4+ T cells and tissue resident macrophages, another latent cellular reservoir to HIV-1. Tissue resident macrophages have been associated with HIV-1 infection since the 1980s, and evidence has continued to grow regarding their role in HIV-1 persistence. Specific biological characteristics play a vital role as to why macrophages are latent cellular reservoirs for HIV-1, and in vitro and in vivo studies exhibit how macrophages contribute to viral persistence in individuals and animals on antiretroviral therapies. In this review, we characterize the role and evolutionary advantages of macrophage reservoirs to HIV-1 and their contribution to HIV-1 persistence. In acknowledging the interplay of HIV-1 and macrophages in the host, we identify reasons why current strategies are incapable of eliminating HIV-1 reservoirs and why efforts must focus on eradicating reservoirs to find a future functional cure.
Keywords: HIV; functional cure; host factors; latency; macrophages; reservoirs.
Copyright © 2021 Hendricks, Cordeiro, Gomes and Stevenson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Host and Viral Factors Influencing Interplay between the Macrophage and HIV-1.J Neuroimmune Pharmacol. 2019 Mar;14(1):33-43. doi: 10.1007/s11481-018-9795-4. Epub 2018 Jul 11. J Neuroimmune Pharmacol. 2019. PMID: 29995208 Free PMC article. Review.
-
Infectious Virus Persists in CD4+ T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2019 Jul 17;93(15):e00065-19. doi: 10.1128/JVI.00065-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31118264 Free PMC article.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
-
Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir.mBio. 2017 Aug 15;8(4):e01186-17. doi: 10.1128/mBio.01186-17. mBio. 2017. PMID: 28811349 Free PMC article.
-
Posttranscriptional Regulation of HIV-1 Gene Expression during Replication and Reactivation from Latency by Nuclear Matrix Protein MATR3.mBio. 2018 Nov 13;9(6):e02158-18. doi: 10.1128/mBio.02158-18. mBio. 2018. PMID: 30425153 Free PMC article.
Cited by
-
Mechanisms underlying the development of type 1 diabetes in ART-treated people living with HIV: an enigmatic puzzle.Front Immunol. 2024 Aug 27;15:1470308. doi: 10.3389/fimmu.2024.1470308. eCollection 2024. Front Immunol. 2024. PMID: 39257582 Free PMC article. Review.
-
Editorial: Direct and Indirect Interactions of HIV With Host Cells.Front Cell Infect Microbiol. 2021 Oct 11;11:771370. doi: 10.3389/fcimb.2021.771370. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34708006 Free PMC article. No abstract available.
-
Proteomic characterization of four subtypes of M2 macrophages derived from human THP-1 cells.J Zhejiang Univ Sci B. 2022 May 15;23(5):407-422. doi: 10.1631/jzus.B2100930. J Zhejiang Univ Sci B. 2022. PMID: 35557041 Free PMC article.
-
HIV-1 cell-to-cell spread overcomes the virus entry block of non-macrophage-tropic strains in macrophages.PLoS Pathog. 2022 May 27;18(5):e1010335. doi: 10.1371/journal.ppat.1010335. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35622876 Free PMC article.
-
Paired ATAC- and RNA-seq offer insight into the impact of HIV on alveolar macrophages: a pilot study.Sci Rep. 2023 Sep 15;13(1):15276. doi: 10.1038/s41598-023-42644-7. Sci Rep. 2023. PMID: 37714998 Free PMC article.
References
-
- Abreu C. M., Price S. L., Shirk E. N., Cunha R. D., Pianowski L. F., Clements J. E., et al. (2014). Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS One 9:e97257. 10.1371/journal.pone.0097257 - DOI - PMC - PubMed
-
- Albright A. V., Shieh J. T., O’connor M. J., Gonzalez-Scarano F. (2000). Characterization of cultured microglia that can be infected by HIV-1. J. Neurovirol. 6(Suppl. 1) S53–S60. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

