Safety and Feasibility of Radiotherapy Plus Camrelizumab for Locally Advanced Esophageal Squamous Cell Carcinoma
- PMID: 33893689
- PMCID: PMC8265339
- DOI: 10.1002/onco.13797
Safety and Feasibility of Radiotherapy Plus Camrelizumab for Locally Advanced Esophageal Squamous Cell Carcinoma
Abstract
Lessons learned: Radiotherapy plus anti-PD-1 antibody as first-line therapy is safe and feasible in locally advanced esophageal squamous cell carcinoma (ESCC). Tumor-infiltrating and peripheral lymphocytes were associated with patient survival. Further studies combining chemoradiotherapy with immunotherapy in locally advanced ESCC and exploration of predictive biomarkers are warranted.
Background: We conducted a phase Ib study of radiotherapy plus programmed cell death protein 1 (PD-1) monoclonal antibody camrelizumab as first-line treatment for locally advanced esophageal squamous cell carcinoma (ESCC).
Methods: We planned to enroll 20 patients with newly diagnosed locally advanced ESCC. Patients received 60 Gy radiation (2.0 Gy/fraction, 5 fractions/week), with camrelizumab (200 mg every 2 weeks) starting with radiotherapy and continuing for 32 weeks (i.e., for 16 cycles). The primary endpoints were safety and feasibility. Secondary endpoints were rates of radiologic and pathologic response, overall survival (OS), and progression-free survival (PFS). Study data were collected by the week during radiotherapy (RT), every month during the maintenance camrelizumab treatment, and every 3 months after treatment. Tumor microenvironment and peripheral blood were monitored at baseline and after 40 Gy radiation for association with efficacy.
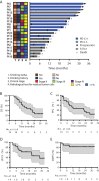
Results: Twenty patients were enrolled and received treatment. One patient (patient 10) was excluded upon discovery of a second tumor in the bladder during treatment, leaving 19 patients for analysis. Toxicity was deemed tolerable. Fourteen (74%) patients had assessed objective response. At a median follow-up time of 31.0 months (95% confidence interval [CI], 27.0-35.1), median OS and PFS times were 16.7 months (95% CI, 5.9-27.9) and 11.7 months (95% CI, 0-30.3), respectively. OS and PFS rates at 24 months were 31.6% and 35.5%, respectively. Kaplan-Meier analysis revealed associations between the following factors and OS/PFS: tumor programmed cell death ligand 1 (PD-L1) expression, PD-1+ CD8+ , PD-1+ CD4+ T cells, and PD-L1+ CD4+ T cells; peripheral blood CD4+ , CD8+ , CD4+ regulatory T cells, and their subsets.
Conclusion: Radiotherapy plus camrelizumab had manageable toxicity and antitumor efficacy for locally advanced ESCC. Several biomarkers were associated with clinical benefit and deserve further study.
Keywords: Camrelizumab; Esophageal cancer; Immunotherapy; PD-1; Radiotherapy.
© AlphaMed Press; the data published online to support this summary are the property of the authors.
Figures





Similar articles
-
Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study.Oncoimmunology. 2021 Sep 28;10(1):1971418. doi: 10.1080/2162402X.2021.1971418. eCollection 2021. Oncoimmunology. 2021. PMID: 34616588 Free PMC article. Clinical Trial.
-
Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma.Cancer Commun (Lond). 2020 Dec;40(12):711-720. doi: 10.1002/cac2.12119. Epub 2020 Dec 12. Cancer Commun (Lond). 2020. PMID: 33314747 Free PMC article. Clinical Trial.
-
Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study.World J Surg Oncol. 2021 Nov 22;19(1):333. doi: 10.1186/s12957-021-02446-5. World J Surg Oncol. 2021. PMID: 34809658 Free PMC article.
-
Experience With Anti-PD-1 Antibody, Camrelizumab, Monotherapy for Biliary Tract Cancer Patients and Literature Review.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820979703. doi: 10.1177/1533033820979703. Technol Cancer Res Treat. 2020. PMID: 33308041 Free PMC article. Review.
-
Salvage camrelizumab plus apatinib for relapsed esophageal neuroendocrine carcinoma after esophagectomy: a case report and review of the literature.Cancer Biol Ther. 2020 Nov 1;21(11):983-989. doi: 10.1080/15384047.2020.1829265. Epub 2020 Oct 23. Cancer Biol Ther. 2020. PMID: 33092443 Free PMC article. Review.
Cited by
-
Expanding the role of combined immunochemotherapy and immunoradiotherapy in the management of head and neck cancer (Review).Oncol Lett. 2023 Jul 17;26(3):372. doi: 10.3892/ol.2023.13958. eCollection 2023 Sep. Oncol Lett. 2023. PMID: 37965160 Free PMC article. Review.
-
CD155 Cooperates with PD-1/PD-L1 to Promote Proliferation of Esophageal Squamous Cancer Cells via PI3K/Akt and MAPK Signaling Pathways.Cancers (Basel). 2022 Nov 15;14(22):5610. doi: 10.3390/cancers14225610. Cancers (Basel). 2022. PMID: 36428703 Free PMC article.
-
Radiotherapy remodels the tumor microenvironment for enhancing immunotherapeutic sensitivity.Cell Death Dis. 2023 Oct 13;14(10):679. doi: 10.1038/s41419-023-06211-2. Cell Death Dis. 2023. PMID: 37833255 Free PMC article. Review.
-
Immune Subtypes in LUAD Identify Novel Tumor Microenvironment Profiles With Prognostic and Therapeutic Implications.Front Immunol. 2022 Jun 3;13:877896. doi: 10.3389/fimmu.2022.877896. eCollection 2022. Front Immunol. 2022. PMID: 35720373 Free PMC article.
-
Long-term efficacy and predictors of pembrolizumab-based regimens in patients with advanced esophageal cancer in the real world.World J Gastroenterol. 2023 Nov 7;29(41):5641-5656. doi: 10.3748/wjg.v29.i41.5641. World J Gastroenterol. 2023. PMID: 38077159 Free PMC article.
References
-
- Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced esophageal cancer: Long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation therapy oncology group. JAMA 1999;281:1623–1627. - PubMed
-
- Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (Radiation Therapy Oncology Group 94‐05) phase III trial of combined‐modality therapy for esophageal cancer: High‐dose versus standard‐dose radiation therapy. J Clin Oncol 2002;20:1167–1174. - PubMed
-
- Okawa T, Kita M, Tanaka M et al. Results of radiotherapy for inoperable locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys 1989;17:49–54. - PubMed
-
- Wu KL, Chen GY, Xu ZY et al. Three‐dimensional conformal radiation therapy for squamous cell carcinoma of the esophagus: A prospective phase I/II study. Radiother Oncol 2009;93:454–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

