Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39)
- PMID: 33891472
- PMCID: PMC8189598
- DOI: 10.1200/JCO.21.00306
Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39)
Erratum in
-
Erratum.J Clin Oncol. 2021 Jul 20;39(21):2420. doi: 10.1200/JCO.21.01441. J Clin Oncol. 2021. PMID: 34265225 Free PMC article. No abstract available.
Abstract
Purpose: To evaluate the addition of the humanized monoclonal antiprogrammed death ligand-1 (PD-L1) antibody, atezolizumab, to platinum-based chemotherapy and bevacizumab in newly diagnosed stage III or IV ovarian cancer (OC).
Methods: This multicenter placebo-controlled double-blind randomized phase III trial (ClinicalTrials.gov identifier: NCT03038100) enrolled patients with newly diagnosed untreated International Federation of Gynecology and Obstetrics (FIGO) stage III or IV OC who either had undergone primary cytoreductive surgery with macroscopic residual disease or were planned to receive neoadjuvant chemotherapy and interval surgery. Patients were stratified by FIGO stage, Eastern Cooperative Oncology Group performance status, tumor immune cell PD-L1 staining, and treatment strategy and randomly assigned 1:1 to receive 3-weekly cycles of atezolizumab 1,200 mg or placebo (day 1, cycles 1-22), with paclitaxel plus carboplatin (day 1, cycles 1-6) plus bevacizumab 15 mg/kg (day 1, cycles 2-22), omitting perioperative bevacizumab in neoadjuvant patients. The co-primary end points were investigator-assessed progression-free survival and overall survival in the intention-to-treat and PD-L1-positive populations.
Results: Between March 8, 2017, and March 26, 2019, 1,301 patients were enrolled. The median progression-free survival was 19.5 versus 18.4 months with atezolizumab versus placebo, respectively (hazard ratio, 0.92; 95% CI, 0.79 to 1.07; stratified log-rank P = .28), in the intention-to-treat population and 20.8 versus 18.5 months, respectively (hazard ratio, 0.80; 95% CI, 0.65 to 0.99; P = .038), in the PD-L1-positive population. The interim (immature) overall survival results showed no significant benefit from atezolizumab. The most common grade 3 or 4 adverse events were neutropenia (21% with atezolizumab v 21% with placebo), hypertension (18% v 20%, respectively), and anemia (12% v 12%).
Conclusion: Current evidence does not support the use of immune checkpoint inhibitors in newly diagnosed OC. Insight from this trial should inform further evaluation of immunotherapy in OC.
Conflict of interest statement
Figures
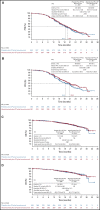

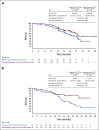
Comment in
-
Immune Checkpoint Inhibitors in Ovarian Cancer: Can We Bridge the Gap Between IMagynation and Reality?J Clin Oncol. 2021 Jun 10;39(17):1833-1838. doi: 10.1200/JCO.21.00571. Epub 2021 Apr 23. J Clin Oncol. 2021. PMID: 33891471 No abstract available.
Similar articles
-
Atezolizumab Combined With Bevacizumab and Platinum-Based Therapy for Platinum-Sensitive Ovarian Cancer: Placebo-Controlled Randomized Phase III ATALANTE/ENGOT-ov29 Trial.J Clin Oncol. 2023 Oct 20;41(30):4768-4778. doi: 10.1200/JCO.23.00529. Epub 2023 Aug 29. J Clin Oncol. 2023. PMID: 37643382 Free PMC article. Clinical Trial.
-
Overall survival and patient-reported outcome results from the placebo-controlled randomized phase III IMagyn050/GOG 3015/ENGOT-OV39 trial of atezolizumab for newly diagnosed stage III/IV ovarian cancer.Gynecol Oncol. 2023 Oct;177:20-31. doi: 10.1016/j.ygyno.2023.06.018. Epub 2023 Aug 23. Gynecol Oncol. 2023. PMID: 37625235 Free PMC article. Clinical Trial.
-
First-line bevacizumab plus chemotherapy in Chinese patients with stage III/IV epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer: a phase III randomized controlled trial.J Gynecol Oncol. 2024 Sep;35(5):e99. doi: 10.3802/jgo.2024.35.e99. Epub 2024 Apr 22. J Gynecol Oncol. 2024. PMID: 38872480 Free PMC article. Clinical Trial.
-
Expert consensus: Profiling and management of advanced or metastatic epithelial ovarian cancer.Rev Colomb Obstet Ginecol. 2024 Jun 14;75(1):4094. doi: 10.18597/rcog.4094. Rev Colomb Obstet Ginecol. 2024. PMID: 39013199 Free PMC article. English, Spanish.
-
Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer.Future Oncol. 2020 Mar;16(7):225-246. doi: 10.2217/fon-2019-0042. Epub 2019 Nov 20. Future Oncol. 2020. PMID: 31746224 Free PMC article. Review.
Cited by
-
Immune-related cardiovascular toxicities of PD-1/PD-L1 inhibitors in solid tumors: an updated systematic review and meta-analysis.Front Immunol. 2024 Jan 22;15:1255825. doi: 10.3389/fimmu.2024.1255825. eCollection 2024. Front Immunol. 2024. PMID: 38318172 Free PMC article.
-
Targeting the immune microenvironment for ovarian cancer therapy.Front Immunol. 2023 Dec 18;14:1328651. doi: 10.3389/fimmu.2023.1328651. eCollection 2023. Front Immunol. 2023. PMID: 38164130 Free PMC article. Review.
-
Therapeutic Strategies Focused on Cancer-Associated Hypercoagulation for Ovarian Clear Cell Carcinoma.Cancers (Basel). 2022 Apr 24;14(9):2125. doi: 10.3390/cancers14092125. Cancers (Basel). 2022. PMID: 35565252 Free PMC article. Review.
-
Bevacizumab with Chemotherapy as a First-Line Treatment for Advanced Ovarian Cancer in a Serbian Cohort.Medicina (Kaunas). 2022 Apr 27;58(5):607. doi: 10.3390/medicina58050607. Medicina (Kaunas). 2022. PMID: 35630024 Free PMC article.
-
Immunology and Immune Checkpoint Inhibition in Ovarian Cancer - Current Aspects.Geburtshilfe Frauenheilkd. 2021 Oct;81(10):1128-1144. doi: 10.1055/a-1475-4335. Epub 2021 Jul 6. Geburtshilfe Frauenheilkd. 2021. PMID: 34629492 Free PMC article.
References
-
- Ferlay J Colombet M Soerjomataram I, et al. : Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144:1941-1953, 2019 - PubMed
-
- Burger RA Brady MF Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 - PubMed
-
- Perren TJ Swart AM Pfisterer J, et al. : A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484-2496, 2011 - PubMed
-
- Moore K Colombo N Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 - PubMed
-
- Ray-Coquard I Pautier P Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

